Menopausal Hormone Replacement Therapy Basics
Rebecca Clemson, ND
After years of unfounded media fueled fear following the results of the Women’s Health Initiative (WHI), menopausal replacement hormone therapy (MHRT) is seeing renewed interest by providers and patients alike. Though lingering misconceptions surrounding MHRT remain, research (including the WHI) has established that for many women MHRT is safe and beneficial. Still, many providers find MHRT overwhelming and conflicting information regarding its safety persists. Having foundational knowledge of the benefits and risks of MHRT is important not only to empower providers but also help educate patients in making informed decisions about their health.
This article will review the basics of MHRT including when to consider MHRT, the benefits, risks, contraindications, and monitoring MHRT. The focus will be on estradiol, progesterone, and DHEA hormone replacement.
MHRT Indications
Menopause is diagnosed one year after the last menstrual cycle. This typically happens for women between the ages of 45 and 55. Menopause is characterized by a dramatic decline in estrogen and progesterone levels, which leads to the myriad of symptoms. Menopausal hormone replacement therapy can bring great relief to women with menopausal related symptoms by replacing low hormones. In general, MHRT refers to estrogen and progestogen therapy, with the preferred forms being bioidentical estradiol and progesterone. While MHRT often begins with the onset of menopause, it can be utilized during perimenopause as well.
Indications to initiate MHRT include:
- Significant vasomotor symptoms (VMS) that disrupt activities of daily living.
- Symptoms NOT controlled to satisfaction by lifestyle changes.
- MHRT is safe given the patient's history.
- In premature menopause, premature ovarian insufficiency (POI), surgical menopause (bilateral oophorectomy) or induced menopause:
MHRT typically centers around estradiol replacement with progesterone added to protect the endometrium.
When to Initiate MHRT
Timing is an important factor when considering initiating MHRT. While women can initiate MHRT at any time to support menopausal related symptoms, there are benefits and risks to consider at each stage. The benefits of MHRT are maximized, and risks are minimized when initiated in women less than 60 years old or within 10 years of menopause. Women see fewer benefits and more risks if they initiate HRT after age 59 or more than 10 years after menopause.
Additionally, there are certain populations where HRT is strongly indicated. HRT is important for young women with early menopause (age 40-45) (whether natural, surgical, or medical) and premature menopause (less than 40 years old) or Premature Ovarian Insufficiency (POI). This helps to normalize risks associated with low estrogen, such as cardiovascular disease and osteoporosis, which are increased in women who go through menopause early.
The appropriateness of HRT should always be individualized, regardless of age.
Contraindications
Patient history must also be taken into account when considering MHRT. There are contraindications for MHRT, including:
- Undiagnosed abnormal vaginal bleeding
- Active deep vein thrombosis (DVT), pulmonary embolism (PE), thrombophlebitis, or history of these conditions
- Active or recent (within the past year) arterial thromboembolic disease (e.g., stroke and myocardial infarction (MI)).
- History of estrogen-sensitive neoplasm (breast, uterine, other)
- Liver disease
Risks
Being aware of and communicating possible risks of MHRT is also important for providers and patients. Although rare, there are risks associated with MHRT. The risks associated with estrogen plus progestogen therapy include Venous thromboembolism (VTE), gallbladder disease, urinary incontinence, stroke, and breast cancer. The risks associated with estrogen only therapy include: VTE, gallbladder disease, urinary incontinence, endometrial hyperplasia*, and endometrial cancer*. *Endometrial hyperplasia and cancer risk can be mitigated by concomitant progestogen therapy.
HRT prescriptions should be individualized to maximize benefits and minimize risk.
The above risks can potentially be mitigated by choosing specific types and delivery methods of MHRT. For example, the rare increased risk of gallbladder disease, stroke, and VTE may be reduced by using transdermal bioidentical estradiol therapy (instead of oral estrogens) and bioidentical progesterone (instead of medroxyprogesterone acetate (MPA)). The rare but increased breast cancer risk may be reduced by using bioidentical progesterone instead of MPA.
A quick note on the risk of breast cancer. Breast cancer is a common concern that patients express when considering MHRT, especially estrogen. The WHI did not find an increased risk in breast cancer for the estrogen only therapy group (they had a decreased risk!). The increased risk that was found with the estrogen and progestogen therapy group (conjugated equine estrogens (CEE) and MPA) was not statistically significant and was still considered rare. Less than one additional case of breast cancer per 1,000 users annually. This is equal to the risk associated with the combination of obesity and a sedentary lifestyle. And less than the risk of two glasses of wine per day.
The rare but increased breast cancer risk may be reduced by using bioidentical progesterone instead of MPA.
In the next few paragraphs, we’ll dive a little deeper into the indications, benefits, and other general information regarding the most common MHRT hormones: estradiol, progesterone, and DHEA.
Estrogen therapy
The decline in estradiol causes many of the symptoms and health conditions associated with menopause. Indications for estrogen therapy include vasomotor symptoms (VMS), vulvovaginal atrophy (VVA), genitourinary syndrome of menopause (GSM), osteoporosis prevention, and hypogonadism of early or premature menopause.
Other benefits of estrogen therapy include:
- Reduction in cardiovascular disease (CVD)
- Bone mineral density improvement
- Sleep support
- Decreased dementia in some populations
- Muscle mass maintenance
- Weight management
- Mood support
- Skin elasticity
- Reduced hair loss
- Decreased joint pain
- Enhanced quality of life
Always review contraindications and potential risks before prescribing (see above). There are many routes of administration (ROA) available for estrogen therapy; however, transdermal, oral, and vaginal ROAs have the most research. Transdermal bioidentical estradiol is the preferred form of estrogen therapy as it can reduce the rare increased risk of gallbladder disease, stroke, and VTE seen with oral estrogens.
Patients with a uterus must pair a progestogen with estrogen MHRT to reduce the risk of endometrial hyperplasia/cancer that can occur with estrogen therapy. Bioidentical progesterone is the preferred form for MHRT.
Progesterone therapy
Since progesterone typically is the first hormone to decline during perimenopause, it is often used as a monotherapy during this stage to reduce heavy periods, reduce VMS due to estrogen fluctuations, and to support sleep and mood. Patients with a uterus must pair a progestogen with estrogen MHRT to reduce the risk of endometrial hyperplasia/cancer that occurs with estrogen therapy. Bioidentical progesterone is the preferred form for MHRT.
Bioidentical progesterone has not been linked to the same breast cancer risk or thrombogenicity as progestins.
Oral micronized progesterone (OMP) is bioidentical progesterone therapy that is FDA approved for the prevention of hyperplasia in postmenopausal women with a uterus. Other benefits of bioidentical progesterone therapy include optimizing bone mineral density, supporting healthy mood, decreasing VMS, and potential brain protective effects. There are contraindications and risks associated with progestogen therapy (see above). Additionally, for OMP, hypersensitivity to progesterone products and an allergy to peanuts (Prometrium specifically, as it contains peanut oil) are additional contraindications.
There are many ROAs available for progesterone therapy; however, only oral micronized progesterone and vaginal progesterone therapy have been shown in studies to stabilize the endometrium for patients on concomitant estrogen therapy.
Transdermal (TD) progesterone in combination with estrogen therapy has NOT been shown to adequately protect the endometrium from hyperplasia.
Androgen therapy: DHEA
Currently, there are no FDA approved testosterone products for women. This does not mean that there is no benefit to testosterone therapy for women; however, in this post the focus will be on DHEA as a common androgen therapy for women. DHEA is a steroid precursor to androgens and 90% of postmenopausal estrogens. The most used forms are oral and vaginal DHEA. Oral DHEA is available as a supplement in the U.S. and is commonly used to support libido, body composition/muscle mass, energy, mood, and sense of well-being. FDA approved Intrarosa is a vaginal DHEA that is indicated for moderate to severe dyspareunia, a symptom of genitourinary syndrome of menopause (GSM).
Contraindications and risks associated with oral DHEA therapy are similar to estrogen therapy (see above). Although contraindications are related to DHEA’s conversion to estrogens and testosterone, the FDA approved vaginal DHEA (Intrarosa) does not significantly impact circulating estradiol levels at the approved dose. Patients with a history of serious estrogen-related diseases such as cancer may still use vaginal DHEA for dyspareunia due to its low-risk profile at approved doses.
Vaginal DHEA may be a better option than vaginal estradiol because it is less likely to result in estradiol exposure in the endometrium. By providing androgens to local vaginal tissue, it may also support sexual function.
Monitoring Hormones with the DUTCH Test
Conventionally, monitoring MHRT has not been recommended due to varying levels of hormones in serum related to timing of dose and route of administration. Dosing MHRT was determined from patient symptomatology and dosing from studies. However, there are benefits to monitoring MHRT, for example: ensuring effective dosing beyond symptom management for bone and cardiovascular health. The DUTCH test also gives insights into downstream hormone metabolites of estrogens and androgens.
Hormone metabolites measured on the DUTCH test may give insight into HRT dosing, symptom management, risk for side effects, and risk for breast cancer.
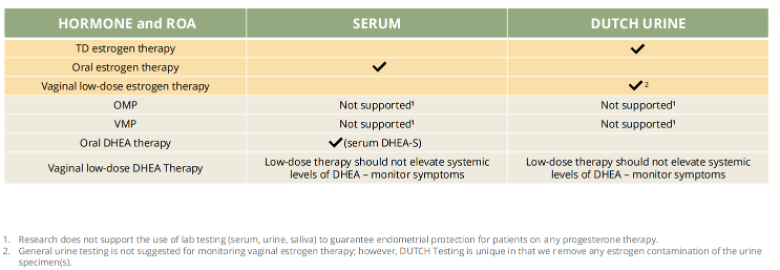
It is suggested to use DUTCH testing for monitoring transdermal and vaginal estrogen therapy, and serum testing for oral estradiol and oral DHEA therapy. The DUTCH Test may be an optimal tool for monitoring circulating estrogen levels when transdermal and vaginal estradiol are supplemented because it shows average hormone levels over a 24-hour window, whereas serum and saliva only show hormone levels at a single point in time.
What about monitoring progesterone therapy? Data suggests that it is not effective to use lab testing (serum, urine, or saliva) to guarantee endometrial protection for patients on any progesterone therapy. Dosing progesterone for endometrial protection with estrogen replacement therapy is based on dosing from studies.
DUTCH Introduction to Menopausal HRT Online Course
We’re excited to announce our new brand-new educational course: Introduction to Menopausal HRT! This course is designed to empower healthcare providers with the essential knowledge and skills needed to effectively support women navigating menopause. The course will provide in depth hormone education, covering association guidelines and MHRT controversies, estrogen, progesterone and androgen therapy and their various routes of administration, dosing, proper baseline evaluation and assessment, and monitoring MHRT with the DUTCH test. The Introduction to Menopausal HRT course is free for all registered DUTCH providers. Enroll in this transformative training to enhance your practice and provide optimal care for women during this pivotal stage of life. If you're not yet a registered DUTCH Provider, sign up today!
TAGS
Hormone Replacement Therapy (HRT)
Estrogen and Progesterone
Postmenopausal Women
Menopause