Get your ticket to DUTCH Fest 2026! Join us March 12-14 in Dallas, TX for cutting-edge hormone education and hands-on learning. Learn more and register today.*
*DUTCH Fest is exclusive to registered DUTCH Providers. Only registered DUTCH Providers can attend.
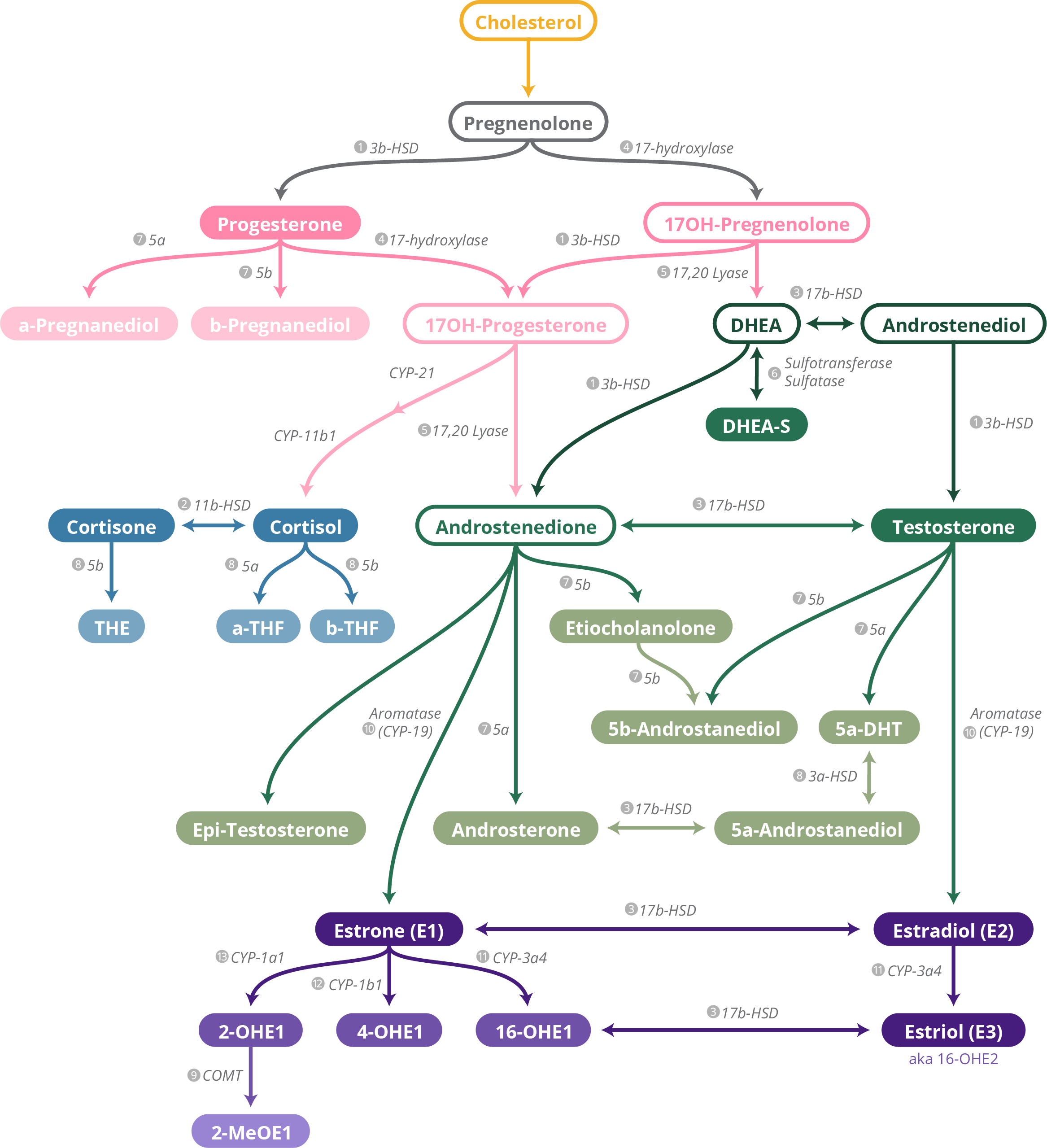
Steroid Pathway
What is the Steroid Pathway?
The DUTCH Steroid Pathway is a summary depicting where hormones come from and how they form through various cells in the body. It includes supplements, nutrients, herbs, and medications shown in the literature to increase or decrease particular enzymes affecting these hormones.
Please note: This is a general steroid pathway and does not specifically differentiate from cells in the ovaries, adrenal glands, or testes.
DISCLAIMER: The information in this handout is provided for informational and educational purposes only and is not medical or treatment advice. Any information and statements regarding dietary or herbal supplements have not been evaluated by the Food and Drug Administration and are not intended to diagnose, treat, cure, or prevent any disease. The use of any information provided in this handout is solely at your own risk.
How do I read the chart?
Begin at the top of the page with cholesterol and follow the arrows downstream to see the conversion of cholesterol into various steroid hormones. Hormones depicted with a solid color bubble are measured by the DUTCH Test, while hormones depicted with an outline are not. The outlined hormones are too far upstream to test directly with our methodology. Instead, we test the downstream metabolites of that hormone.
Hormones are color-coded for convenient reading:

Next to each arrow on the steroid pathway chart is the name of the protein responsible for moving each hormone further downstream. These proteins are important because they can be targeted with lifestyle changes and supplementation to improve symptoms associated hormone imbalances.
Contributing Factors
Use the corresponding number next to a protein to find the list of contributing factors that may be affecting the results of a DUTCH Test.
- Hamden, K., et al., Potential protective effect on key steroidogen sis and metabolic enzymes and sperm abnormalities by fenugreek steroids in testis and epididymis of surviving diabetic rats. Arch Physiol Biochem, 2010. 116(3): p. 146-55.
- Simonian, M.H., ACTH and thyroid hormone regulation of 3 beta-hydroxysteroid dehydrogenase activity in human fetal adrenocortical cells. J Steroid Biochem, 1986. 25(6): p. 1001-6.
- Kaaijk, E.M., et al., Distribution of steroidogenic enzymes involved in androgen synthesis in polycystic ovaries: an immunohistochemical study. Mol Hum Reprod, 2000. 6(5): p. 443-7.
- Deluca, D., et al., Inhibition of 17beta-hydroxysteroid dehydrogenases by phytoestrogens: comparison with other steroid metabolizing enzymes. J Steroid Biochem Mol Biol, 2005. 93(2-5): p. 285-92.
- Zhang, S., et al., Endocrine disruptors of inhibiting testicular 3β-hydroxysteroid dehydrogenase. Chem Biol Interact, 2019. 303: p. 90-97.
- Tomlinson, J.W., et al., Impaired glucose tolerance and insulin resistance are associated with increased adipose 11beta-hydroxysteroid dehydrogenase type 1 expression and elevated hepatic 5alpha-reductase activity. Diabetes, 2008. 57(10): p. 2652-60.
- Stomati, M., et al., Six-month oral dehydroepiandrosterone supplementation in early and late postmenopause. Gynecol Endocrinol, 2000. 14(5): p. 342-63.
- Tsilchorozidou, T., J.W. Honour, and G.S. Conway, Altered cortisol metabolism in polycystic ovary syndrome: insulin enhances 5alpha-reduction but not the elevated adrenal steroid production rates. J Clin Endocrinol Metab, 2003. 88(12): p. 5907-13.
- Prager, N., et al., A randomized, double-blind, placebo-controlled trial to determine the effectiveness of botanically derived inhibitors of 5-alpha-reductase in the treatment of androgenetic alopecia. J Altern Complement Med, 2002. 8(2): p. 143-52.
- Fujita, R., et al., Anti-androgenic activities of Ganoderma lucidum. J Ethnopharmacol, 2005. 102(1): p. 107-12.
- Moradi, H.R., et al., The histological and histometrical effects of Urtica dioica extract on rat's prostate hyperplasia. Vet Res Forum, 2015. 6(1): p. 23-9.
- Wilt, T., et al., Pygeum africanum for benign prostatic hyperplasia. Cochrane Database Syst Rev, 2002(1): p. CD001044.
- Azzouni, F., et al., The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases. Adv Urol, 2012. 2012: p. 530121.
- Westerbacka, J., et al., Body fat distribution and cortisol metabolism in healthy men: enhanced 5beta-reductase and lower cortisol/cortisone metabolite ratios in men with fatty liver. J Clin Endocrinol Metab, 2003. 88(10): p. 4924-31.
- Gambineri, A., et al., Increased clearance of cortisol by 5beta-reductase in a subgroup of women with adrenal hyperandrogenism in polycystic ovary syndrome. J Endocrinol Invest, 2009. 32(3): p. 210-8.
- Ojima, M., et al., [The inhibitory effects of glycyrrhizin and glycyrrhetinic acid on the metabolism of cortisol and prednisolone--in vivo and in vitro studies]. Nihon Naibunpi Gakkai Zasshi, 1990. 66(5): p. 584-96.
- Dube, S., et al., 11β-hydroxysteroid dehydrogenase types 1 and 2 activity in subcutaneous adipose tissue in humans: implications in obesity and diabetes. J Clin Endocrinol Metab, 2015. 100(1): p. E70-6.
- Esteves, C.L., et al., Proinflammatory cytokine induction of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) in human adipocytes is mediated by MEK, C/EBPβ, and NF-κB/RelA. J Clin Endocrinol Metab, 2014. 99(1): p. E160-8.
- Chapman, K., M. Holmes, and J. Seckl, 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev, 2013. 93(3): p. 1139-206.
- Hintzpeter, J., et al., Green tea and one of its constituents, Epigallocatechine-3-gallate, are potent inhibitors of human 11β-hydroxysteroid dehydrogenase type 1. PLoS One, 2014. 9(1): p. e84468.
- Hu, G.X., et al., Curcumin as a potent and selective inhibitor of 11β-hydroxysteroid dehydrogenase 1: improving lipid profiles in high-fat-diet-treated rats. PLoS One, 2013. 8(3): p. e49976.
- Atanasov, A.G., et al., Coffee inhibits the reactivation of glucocorticoids by 11beta-hydroxysteroid dehydrogenase type 1: a glucocorticoid connection in the anti-diabetic action of coffee? FEBS Lett, 2006. 580(17): p. 4081-5.
- Jothie Richard, E., et al., Anti-stress Activity of Ocimum sanctum: Possible Effects on Hypothalamic-Pituitary-Adrenal Axis. Phytother Res, 2016. 30(5): p. 805-14.
- Blum, A., et al., Momordica charantia extract, a herbal remedy for type 2 diabetes, contains a specific 11β-hydroxysteroid dehydrogenase type 1 inhibitor. J Steroid Biochem Mol Biol, 2012. 128(1-2): p. 51-5.
- Hoshiro, M., et al., Comprehensive study of urinary cortisol metabolites in hyperthyroid and hypothyroid patients. Clin Endocrinol (Oxf), 2006. 64(1): p. 37-45.
- Taniyama, M., K. Honma, and Y. Ban, Urinary cortisol metabolites in the assessment of peripheral thyroid hormone action: application for diagnosis of resistance to thyroid hormone. Thyroid, 1993. 3(3): p. 229-33.
- Ueshiba, H., et al., Decreased steroidogenic enzyme 17,20-lyase and increased 17-hydroxylase activities in type 2 diabetes mellitus. Eur J Endocrinol, 2002. 146(3): p. 375-80.
- Nestler, J.E. and D.J. Jakubowicz, Decreases in ovarian cytochrome P450c17 alpha activity and serum free testosterone after reduction of insulin secretion in polycystic ovary syndrome. N Engl J Med, 1996. 335(9): p. 617-23.
- Engelhardt, D., et al., The influence of ketoconazole on human adrenal steroidogenesis: incubation studies with tissue slices. Clin Endocrinol (Oxf), 1991. 35(2): p. 163-8.
- Kossor, D.C. and H.D. Colby, Dose-dependent actions of spironolactone on the inner and outer zones of the guinea pig adrenal cortex. Pharmacology, 1992. 45(1): p. 27-33.
- Hasegawa, E., et al., Effect of polyphenols on production of steroid hormones from human adrenocortical NCI-H295R cells. Biol Pharm Bull, 2013. 36(2): p. 228-37.
- Marti, N., et al., Resveratrol inhibits androgen production of human adrenocortical H295R cells by lowering CYP17 and CYP21 expression and activities. PLoS One, 2017. 12(3): p. e0174224.
- Andric, S.A., et al., Acute effects of polychlorinated biphenyl-containing and -free transformer fluids on rat testicular steroidogenesis. Environ Health Perspect, 2000. 108(10): p. 955-9.
- Kim, S.H., et al., Body Fat Mass Is Associated With Ratio of Steroid Metabolites Reflecting 17,20-Lyase Activity in Prepubertal Girls. J Clin Endocrinol Metab, 2016. 101(12): p. 4653-4660.
- Armanini, D., G. Bonanni, and M. Palermo, Reduction of serum testosterone in men by licorice. N Engl J Med, 1999. 341(15): p. 1158.
- Armanini, D., et al., Licorice reduces serum testosterone in healthy women. Steroids, 2004. 69(11-12): p. 763-6.
- Serafini, P. and R.A. Lobo, The effects of spironolactone on adrenal steroidogenesis in hirsute women. Fertil Steril, 1985. 44(5): p. 595-9.
- Ayub, M. and M.J. Levell, Inhibition of human adrenal steroidogenic enzymes in vitro by imidazole drugs including ketoconazole. J Steroid Biochem, 1989. 32(4): p. 515-24.
- Wang, X., et al., Suppression of rat and human androgen biosynthetic enzymes by apigenin: Possible use for the treatment of prostate cancer. Fitoterapia, 2016. 111: p. 66-72.
- Hu, T., et al., Brown adipose tissue activation by rutin ameliorates polycystic ovary syndrome in rat. J Nutr Biochem, 2017. 47: p. 21-28.
- Sarkola, T., et al., Acute effect of alcohol on androgens in premenopausal women. Alcohol Alcohol, 2000. 35(1): p. 84-90.
- Corbould, A.M., et al., The effect of obesity on the ratio of type 3 17beta-hydroxysteroid dehydrogenase mRNA to cytochrome P450 aromatase mRNA in subcutaneous abdominal and intra-abdominal adipose tissue of women. Int J Obes Relat Metab Disord, 2002. 26(2): p. 165-75.
- Krazeisen, A., et al., Human 17beta-hydroxysteroid dehydrogenase type 5 is inhibited by dietary flavonoids. Adv Exp Med Biol, 2002. 505: p. 151-61.
- Le Bail, J.C., et al., Effects of phytoestrogens on aromatase, 3beta and 17beta-hydroxysteroid dehydrogenase activities and human breast cancer cells. Life Sci, 2000. 66(14): p. 1281-91.
- Abarikwu, S.O. and E.O. Farombi, Quercetin ameliorates atrazine-induced changes in the testicular function of rats. Toxicol Ind Health, 2016. 32(7): p. 1278-85.
- Gérard, C. and K.A. Brown, Obesity and breast cancer - Role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Mol Cell Endocrinol, 2018. 466: p. 15-30.
- Randolph, J.F., et al., The effect of insulin on aromatase activity in isolated human endometrial glands and stroma. Am J Obstet Gynecol, 1987. 157(6): p. 1534-9.
- Watanabe, M. and S. Nakajin, Forskolin up-regulates aromatase (CYP19) activity and gene transcripts in the human adrenocortical carcinoma cell line H295R. J Endocrinol, 2004. 180(1): p. 125-33.
- Sanderson, J.T., et al., Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol Sci, 2004. 82(1): p. 70-9.
- Takeuchi, T., et al., Effect of paeoniflorin, glycyrrhizin and glycyrrhetic acid on ovarian androgen production. Am J Chin Med, 1991. 19(1): p. 73-8.
- Holloway, A.C., et al., Atrazine-induced changes in aromatase activity in estrogen sensitive target tissues. J Appl Toxicol, 2008. 28(3): p. 260-70.
- Lephart, E.D., Modulation of Aromatase by Phytoestrogens. Enzyme Res, 2015. 2015: p. 594656.
- Novaes, M.R., et al., The effects of dietary supplementation with Agaricales mushrooms and other medicinal fungi on breast cancer: evidence-based medicine. Clinics (Sao Paulo), 2011. 66(12): p. 2133-9.
- Satoh, K., et al., Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem Toxicol, 2002. 40(7): p. 925-33.
- Eng, E.T., et al., Suppression of estrogen biosynthesis by procyanidin dimers in red wine and grape seeds. Cancer Res, 2003. 63(23): p. 8516-22.
- Chen, J., et al., The correlation of aromatase activity and obesity in women with or without polycystic ovary syndrome. J Ovarian Res, 2015. 8: p. 11.
- Ayub, M. and M.J. Levell, The inhibition of human prostatic aromatase activity by imidazole drugs including ketoconazole and 4-hydroxyandrostenedione. Biochem Pharmacol, 1990. 40(7): p. 1569-75.
- Rice, S., et al., Dual effect of metformin on growth inhibition and oestradiol production in breast cancer cells. Int J Mol Med, 2015. 35(4): p. 1088-94.
- Richard, S., et al., Differential effects of glyphosate and roundup on human placental cells and aromatase. Environ Health Perspect, 2005. 113(6): p. 716-20.
- Hodges, R.E. and D.M. Minich, Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J Nutr Metab, 2015. 2015: p. 760689.
- Michnovicz, J.J., H. Adlercreutz, and H.L. Bradlow, Changes in levels of urinary estrogen metabolites after oral indole-3-carbinol treatment in humans. J Natl Cancer Inst, 1997. 89(10): p. 718-23.
- Sowers, M.R., et al., Selected diet and lifestyle factors are associated with estrogen metabolites in a multiracial/ethnic population of women. J Nutr, 2006. 136(6): p. 1588-95.
- Lu, L.J., et al., Increased urinary excretion of 2-hydroxyestrone but not 16alpha-hydroxyestrone in premenopausal women during a soya diet containing isoflavones. Cancer Res, 2000. 60(5): p. 1299-305.
- Chen, H.W., et al., The combined effects of garlic oil and fish oil on the hepatic antioxidant and drug-metabolizing enzymes of rats. Br J Nutr, 2003. 89(2): p. 189-200.
- Debersac, P., et al., Induction of cytochrome P450 and/or detoxication enzymes by various extracts of rosemary: description of specific patterns. Food Chem Toxicol, 2001. 39(9): p. 907-18.
- Michnovicz, J.J. and R.A. Galbraith, Effects of exogenous thyroxine on C-2 and C-16 alpha hydroxylations of estradiol in humans. Steroids, 1990. 55(1): p. 22-6.
- Peters, L.P. and R.W. Teel, Effect of high sucrose diet on cytochrome P450 1A and heterocyclic amine mutagenesis. Anticancer Res, 2003. 23(1A): p. 399-403.
- Mahabir, S., et al., Effects of low-to-moderate alcohol supplementation on urinary estrogen metabolites in postmenopausal women in a controlled feeding study. Cancer Med, 2017. 6(10): p. 2419-2423.
- Licznerska, B., et al., Resveratrol and its methoxy derivatives modulate the expression of estrogen metabolism enzymes in breast epithelial cells by AhR down-regulation. Mol Cell Biochem, 2017. 425(1-2): p. 169-179.
- Smerdová, L., et al., Upregulation of CYP1B1 expression by inflammatory cytokines is mediated by the p38 MAP kinase signal transduction pathway. Carcinogenesis, 2014. 35(11): p. 2534-43.
- Li, M.Y., et al., Estrogen receptor alpha promotes smoking-carcinogen-induced lung carcinogenesis via cytochrome P450 1B1. J Mol Med (Berl), 2015. 93(11): p. 1221-33.
- Jaramillo, I.C., et al., Effects of fuel components and combustion particle physicochemical properties on toxicological responses of lung cells. J Environ Sci Health A Tox Hazard Subst Environ Eng, 2018. 53(4): p. 295-309.
- Doostdar, H., M.D. Burke, and R.T. Mayer, Bioflavonoids: selective substrates and inhibitors for cytochrome P450 CYP1A and CYP1B1. Toxicology, 2000. 144(1-3): p. 31-8.
- Whitten, D.L., et al., The effect of St John's wort extracts on CYP3A: a systematic review of prospective clinical trials. Br J Clin Pharmacol, 2006. 62(5): p. 512-26.
- Bradlow, H.L., et al., Effects of pesticides on the ratio of 16 alpha/2-hydroxyestrone: a biologic marker of breast cancer risk. Environ Health Perspect, 1995. 103 Suppl 7: p. 147-50.
- Luckert, C., et al., Polycyclic aromatic hydrocarbons stimulate human CYP3A4 promoter activity via PXR. Toxicol Lett, 2013. 222(2): p. 180-8.
- Wu, W.H., et al., Estrogenic effect of yam ingestion in healthy postmenopausal women. J Am Coll Nutr, 2005. 24(4): p. 235-43.
- Dresser, G.K., et al., Evaluation of peppermint oil and ascorbyl palmitate as inhibitors of cytochrome P4503A4 activity in vitro and in vivo. Clin Pharmacol Ther, 2002. 72(3): p. 247-55.
- Niwa, T., Y. Imagawa, and H. Yamazaki, Drug interactions between nine antifungal agents and drugs metabolized by human cytochromes P450. Curr Drug Metab, 2014. 15(7): p. 651-79.
- Jiang, H., et al., Human catechol-O-methyltransferase down-regulation by estradiol. Neuropharmacology, 2003. 45(7): p. 1011-8.
- Ho, P.W., et al., Effects of plasticisers and related compounds on the expression of the soluble form of catechol-O-methyltransferase in MCF-7 cells. Curr Drug Metab, 2008. 9(4): p. 276-9.
- Blum, K., et al., Manipulation of catechol-O-methyl-transferase (COMT) activity to influence the attenuation of substance seeking behavior, a subtype of Reward Deficiency Syndrome (RDS), is dependent upon gene polymorphisms: a hypothesis. Med Hypotheses, 2007. 69(5): p. 1054-60.
- van Duursen, M.B., et al., Phytochemicals inhibit catechol-O-methyltransferase activity in cytosolic fractions from healthy human mammary tissues: implications for catechol estrogen-induced DNA damage. Toxicol Sci, 2004. 81(2): p. 316-24.
- Sehirli, A.O., et al., St. John's wort may ameliorate 2,4,6-trinitrobenzenesulfonic acid colitis off rats through the induction of pregnane X receptors and/or P-glycoproteins. J Physiol Pharmacol, 2015. 66(2): p. 203-14.
- Pascussi, J.M., et al., Dexamethasone induces pregnane X receptor and retinoid X receptor-alpha expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol, 2000. 58(2): p. 361-72.
- Zhou, H. and P.B. Hylemon, Bile acids are nutrient signaling hormones. Steroids, 2014. 86: p. 62-8.
- Ding, X. and J.L. Staudinger, Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther, 2005. 312(2): p. 849-56.
- Mueller, J.W., et al., The Regulation of Steroid Action by Sulfation and Desulfation. Endocr Rev, 2015. 36(5): p. 526-63.
- Kim, M.S., et al., Suppression of DHEA sulfotransferase (Sult2A1) during the acute-phase response. Am J Physiol Endocrinol Metab, 2004. 287(4): p. E731-8.
- Al-Dujaili, E.A., et al., Liquorice and glycyrrhetinic acid increase DHEA and deoxycorticosterone levels in vivo and in vitro by inhibiting adrenal SULT2A1 activity. Mol Cell Endocrinol, 2011. 336(1-2): p. 102-9.
We are here to support you on your journey
With these metabolites, DUTCH Test provides the industry’s most extensive profile of sex and adrenal hormones. Additionally, the daily (diurnal) pattern of free cortisol is included, along with melatonin (6-OHMS), 8-OHdG, and nine organic acids. This unique combination of clinical information is not available through any other testing method.
8-OHdG: Our Oxidative Stress Biomarker
Oxidative stress refers to cellular damage or DNA damage. The body needs healthy and robust cells and DNA. Cell-signaling influences blood sugar, hormone production, detoxification, the creation of new cells, etc. Oxidation is like aging. If cells age too quickly, the body is unable to heal and recover well.
8-OHdG measures the effect of endogenous oxidative stress and DNA damage, it is also used to estimate the DNA damage in humans after exposure to cancer-causing agents such as tobacco smoke, asbestos fibers, heavy metals, and polycyclic aromatic hydrocarbons.
What Happens?
When local antioxidant systems fail, oxidative damage permanently occurs to lipids of cellular membranes, proteins, and DNA. In nuclear and mitochondrial DNA, 8-OHdG is predominantly formed due to free radical-induced oxidative (pro-mutative) lesions.
Studies and Cancer:
60 women with malignant tumors in a breast cancer study1and 82 men in a prostate cancer study showed 8-OHdG levels significantly higher than controls2. Levels did not decrease with prostatectomy but did decrease with androgen suppression hormone therapy.
Additional Information:
Orange juice (but not pomegranate, apple, grapefruit or cranberry) reduced oxidative stress measured by 8-OHdG3. Taking micronutrient and mineral supplements with antioxidants improved 8-OHdG in people who otherwise did not eat vegetables4. When renoprotective effects of berberine were measured by 8-OHdG in patients with both hypertension and type 2 diabetes, berberine reduced 8-OHdG among other measures5. 8-OHdG increased in the kidney and liver with a copper releasing implant, and researchers supposed that this might also happen with copper IUDs in humans6. Smokers who have high 8-OHdG can lower it by taking moderate amounts of fish oil with combined EPA/DHA7. Urinary BPA increases associated with urinary 8-OHdG increase8. Urinary methylparaben (MP) and ethylparaben (EP) increase along with 8-OHdG in pregnant women and their infants9.
See below for the references annotated above:
- Kuo HW, Chou SY, Hu TW, Wu FY, Chen DJ. 2007. Urinary 8-hydroxy-2-deoxyguanosine (8OHdG) and genetic polymorphisms in breast cancer patients. Mutation Research Genetic Toxicology and Environmental Mutagenesis. 631(1):62-68.
- Miyake H, Hara I, Kamidono S, Eto H. 2004. Oxidative DNA Damage in Patients with Prostate Cancer and its Response to Treatment. 171(4):15331536.
- Hyson DA. A review and critical analysis of the scientific literature related to 100% fruit juice and human health. Adv Nutr 2015, Jan;6(1):37-51.
- Kim YJ, Ahn YH, Lim Y, Kim JY, Kim J, Kwon O. Daily nutritional dose supplementation with antioxidant nutrients and phytochemicals improves DNA and LDL stability: A double-blind, randomized, and placebo-controlled trial. Nutrients 2013, Dec 18;5(12):5218-32.
- Dai P, Wang J, Lin L, Zhang Y, Wang Z. Renoprotective effects of berberine as adjuvant therapy for hypertensive patients with type 2 diabetes mellitus: Evaluation via biochemical markers and color doppler ultrasonography. Exp Ther Med 2015, Sep;10(3):869-76.
- Toyokuni S, Sagripanti JL. Increased 8-hydroxydeoxyguanosine in kidney and liver of rats continuously exposed to copper. Toxicol Appl Pharmacol 1994, May;126(1):91-7.
- Ghorbanihaghjo A, Safa J, Alizadeh S, Argani H, Rashtchizadeh N, Taghinia MV, Abbasi MM. Protective effect of fish oil supplementation on DNA damage induced by cigarette smoking. J Health Popul Nutr 2013, Sep;31(3):343-9.
- Watkins DJ, Ferguson KK, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int J Hyg Environ Health 2015, Mar;218(2):212-9.
- Kang S, Kim S, Park J, Kim HJ, Lee J, Choi G, et al. Urinary paraben concentrations among pregnant women and their matching newborn infants of korea, and the association with oxidative stress biomarkers. Sci Total Environ 2013, Sep 1;461-462:214-21.