The Role of Adrenals in PCOS
by Kelly Ruef, ND
It’s estimated that 20-30% of women diagnosed with polycystic ovary syndrome (PCOS) have excess DHEA-S, indicating some degree of adrenal involvement in their condition. Considering this staggering statistic and knowing that PCOS is one of the most common endocrine disorders in women of reproductive age, it seems worthwhile for providers to learn more about adrenal PCOS.
It is also valuable to assess how providers can best utilize lab testing to identify adrenal PCOS. For example, the DUTCH Test measures seven urinary androgen metabolites that often provide greater insight into a patient’s androgen status than serum markers alone.
Let’s explore the etiology (cause) of adrenal PCOS and how to evaluate for adrenal PCOS with the DUTCH Test.
Defining PCOS
PCOS stands for polycystic ovary syndrome. It is one of the most common endocrine disorders in women of reproductive age. Women with PCOS may experience:
- Irregular or absent cycles
- Unwanted facial and body hair growth
- Scalp hair loss
- Acne
- Anxiety and depression
- Difficulty conceiving
- Weight gain

Adrenal Involvement in PCOS
The etiology of adrenal PCOS is not 100% clear, but there is some knowledge on the subject that can inform a functional medicine approach to care. Adrenal PCOS may be due to a combination of factors, including hyperresponsive adrenals, genetic factors, premature adrenarche, and stress in general.
Hyperresponsive Adrenals
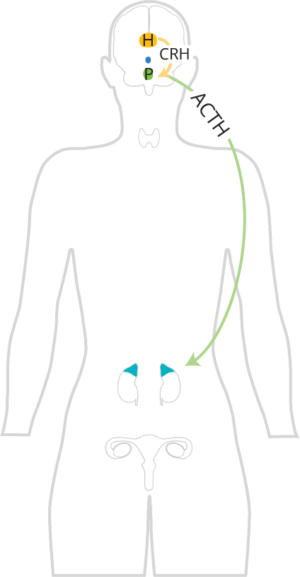
Adrenocorticotropic hormone (ACTH) promotes the release of cortisol and androgens from the adrenal cortex. It specifically promotes the release of adrenal androgens, such as DHEA, DHEA-S, androstenedione, and testosterone from the zona reticularis.
In women with an adrenal component to their PCOS, their zona reticularis is hyperresponsive to the ACTH signal from the pituitary. This is significant, because even if they have normal ACTH levels and no HPA axis dysfunction, their adrenals may still overproduce androgens.
Genetic Factors
Genetics could contribute to the etiology, signs, and symptoms of PCOS, as elevated androgens (DHEA-S, androstenedione) have a high heredity. Genetic variants in SULT2A1 and PAPS synthase 2 (PAPSS2) deficiency can result in lower DHEA-S, and potentially more conversion of DHEA to androstenedione, testosterone, and 5a-DHT.
Remember, testosterone and 5a-DHT are active, whereas DHEA, DHEA-S, and androstenedione have little to no activity on the androgen receptors. SULT2A1 sulfates DHEA to DHEA-S, and PAPSS2 provides the universal sulfate donor (PAPS) to all sulfotransferases in the human body, including SULT2A1. Genetic variants likely only account for a small fraction of women suffering from adrenal PCOS.
Premature Adrenarche
Adrenarche is when the adrenal gland goes through puberty. Adrenarche is considered premature if it occurs in girls younger than eight years old or in boys younger than nine years old. During adrenarche, the zona reticularis (the part of the adrenal glands that produces androgens) develops, and DHEA and DHEA-S levels rise. ACTH and cortisol levels do not rise during adrenarche; thus, the increase in DHEA and DHEA-S is not due to a change in ACTH signaling from the brain. Premature adrenarche is 15–20% more prevalent in women with PCOS.
Stress
As stress increases ACTH signaling, and ACTH promotes the release of cortisol and androgens from the adrenal glands, increased stress may lead to elevated adrenal androgens. Stress can be real or perceived, and there are many different types of stressors (psychological, exercise, acute pain, acute inflammation, acute infection, etc.). Therefore, stress reduction techniques and eliminating or reducing other adrenal stressors are of utmost importance.
Insulin Resistance
It is well known that insulin resistance increases ovarian production of androgens, however, it likely plays a more limited role in the elevated androgens seen with adrenal PCOS.
Identifying Adrenal Involvement in PCOS Through DUTCH Testing
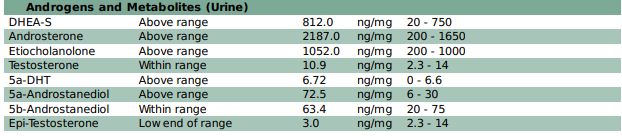
The DUTCH Test measures seven androgen urinary metabolites that may be helpful when assessing adrenal involvement in PCOS. It often provides more insight into androgen levels than serum testing, which is significant as sometimes elevated androgens are not identified in the serum of women with PCOS.
Androgens may be coming from the ovaries if the following markers are elevated:
- Testosterone
- 5a-DHT
- 5a-androstanediol
- 5b-androstanediol
Androgens may be coming from the adrenals if the following markers are elevated:
- DHEA-S
- Etiocholanolone
- Androsterone
Please be advised that DHEA can influence testosterone metabolite levels, and testosterone can influence etiocholanolone and androsterone levels to some degree, so this is only a guideline. The DUTCH Test is not diagnostic and cannot 100% differentiate between ovarian and adrenal androgen production.
5a-Androstanediol: A Better Indicator of 5a-DHT Levels in Target Tissues
In target tissues, testosterone is activated to 5a-DHT by the enzyme 5a-reductase. 5a-DHT is three times more potent than testosterone and is made and metabolized locally in the tissues to 5a-androstanediol. Urinary 5a-androstanediol tends to circulate better than 5a-DHT and may be a better marker of target tissue 5a-DHT activity than urinary (or serum) 5a-DHT itself. Please pay close attention to it when evaluating a patient’s DUTCH Test results.
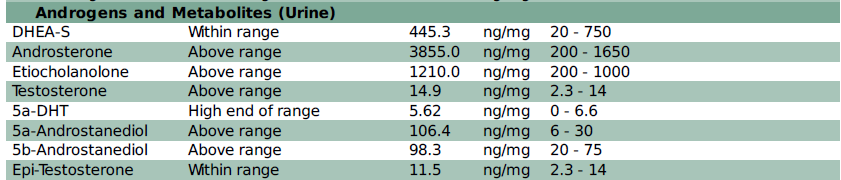
DUTCH Test Example #1
In the following DUTCH Complete PCOS example, you’ll see that this 30-year-old female with hirsutism and hair loss has elevated DHEA metabolites but does not have elevated cortisol. She likely has an adrenal component to her PCOS, and her elevated DHEA metabolites may be due to hyperresponsive adrenals, not increased ACTH signaling.
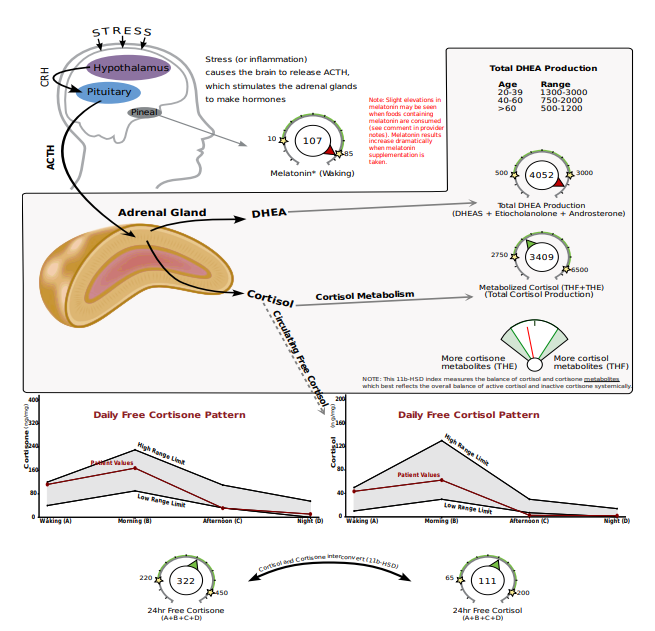
Her downstream testosterone alpha metabolites, 5a-DHT and 5a-androstanediol, are also elevated so it’s possible (and likely) that her ovaries and adrenals are both contributing to the androgen excess. PCOS that involves excess androgen production from the adrenals only (no ovarian involvement) only accounts for 3% of cases.
DUTCH Test Example #2 (NOTE: ACCESSION #783594)
In this next DUTCH Complete PCOS example, you’ll see that this 32-year-old patient’s downstream DHEA metabolites, etiocholanolone, and androsterone are elevated. In addition (and contrary to the previous example) her cortisol is also elevated, indicating possible HPA-axis dysfunction. Stress reduction and adrenal support may be especially important in this case.
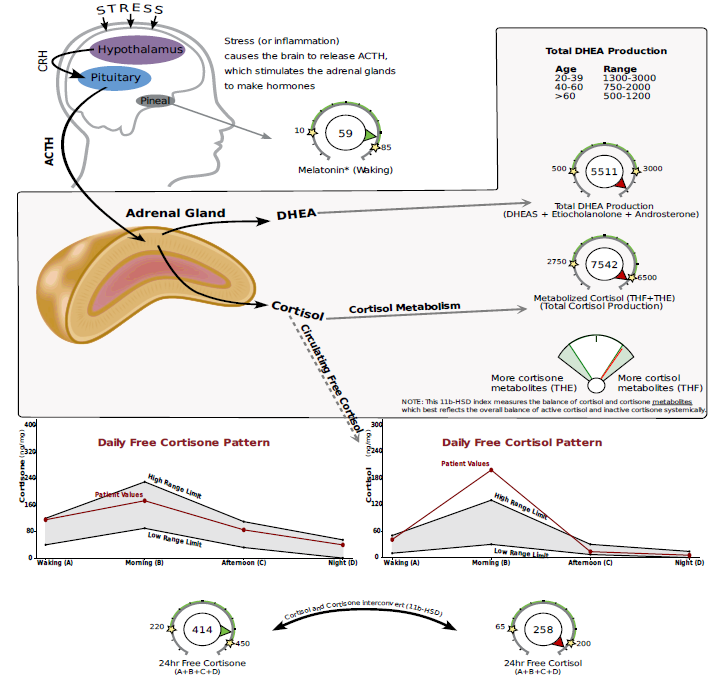
Testosterone and the downstream metabolites of testosterone are also elevated, indicating ovarian involvement is also likely.
In summary, PCOS is one of the most common endocrine disorders in women of reproductive age, affecting up to 15% of the population. The adrenals can play a role in the androgen excess picture, and it’s even estimated that 20-30% of women with PCOS have adrenal involvement. The etiology of adrenal PCOS is unclear but likely involves hyperresponsive adrenals, genetic factors, premature adrenarche, and stress.
The DUTCH Test is comprehensive in that it measures seven androgen metabolites in the urine. It often provides more insight into androgen levels than serum testing, which is significant as elevated androgens are not always identified in the serum of women with PCOS. When investigating whether the HPA axis may be contributing to your patient’s PCOS symptoms, consider ordering a DUTCH Complete or DUTCH Plus panel to gain more insight into androgen levels and cortisol patterns.
References
- Adams JM, Taylor AE, Crowley WF, Jr., Hall JE. Polycystic ovarian morphology with regular ovulatory cycles: insights into the pathophysiology of polycystic ovarian syndrome. J Clin Endocrinol Metab. 2004;89(9):4343-50. Epub 2004/09/10. doi: 10.1210/jc.2003-031600. PubMed PMID: 15356031.
- Barbieri R, et. al. Clinical manifestations of polycystic ovary syndrome in adults. In: UpToDate, Crowley WF (Ed), UpToDate, Waltham, MA. (Accessed on June 28th 2023.)
- Che Y, Yu J, Li YS, Zhu YC, Tao T. Polycystic Ovary Syndrome: Challenges and Possible Solutions. J Clin Med. 2023;12(4). Epub 2023/02/26. doi: 10.3390/jcm12041500. PubMed PMID: 36836035; PubMed Central PMCID: PMCPMC9967025.
- Franks S, Webber LJ, Goh M, Valentine A, White DM, Conway GS, et al. Ovarian morphology is a marker of heritable biochemical traits in sisters with polycystic ovaries. J Clin Endocrinol Metab. 2008;93(9):3396-402. Epub 2008/06/19. doi: 10.1210/jc.2008-0369. PubMed PMID: 18559912.
- Goodarzi MO, Carmina E, Azziz R. DHEA, DHEAS and PCOS. J Steroid Biochem Mol Biol. 2015;145:213-25. Epub 2014/07/11. doi: 10.1016/j.jsbmb.2014.06.003. PubMed PMID: 25008465.
- Ibanez L, Dimartino-Nardi J, Potau N, Saenger P. Premature adrenarche--normal variant or forerunner of adult disease? Endocr Rev. 2000;21(6):671-96. Epub 2001/01/02. doi: 10.1210/edrv.21.6.0416. PubMed PMID: 11133068.
- Keleştimur F, Şahin Y. Alternate pathway 17,20-lyase enzyme activity in the adrenals is enhanced in patients with polycystic ovary syndrome. Fertility and Sterility. 1999;71(6):1075-8. doi: 10.1016/s0015-0282(99)00118-1.
- Kumar A, Woods KS, Bartolucci AA, Azziz R. Prevalence of adrenal androgen excess in patients with the polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2005;62(6):644-9. Epub 2005/06/10. doi: 10.1111/j.1365-2265.2005.02256.x. PubMed PMID: 15943823.
- Rosenfield RL. Current concepts of polycystic ovary syndrome pathogenesis. Curr Opin Pediatr. 2020;32(5):698-706. Epub 2020/09/06. doi: 10.1097/MOP.0000000000000945. PubMed PMID: 32889963; PubMed Central PMCID: PMCPMC7774867.
- Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev. 2016;37(5):467-520. Epub 2016/07/28. doi: 10.1210/er.2015-1104. PubMed PMID: 27459230; PubMed Central PMCID: PMCPMC5045492.
- Smet ME, McLennan A. Rotterdam criteria, the end. Australas J Ultrasound Med. 2018;21(2):59-60. Epub 2018/05/17. doi: 10.1002/ajum.12096. PubMed PMID: 34760503; PubMed Central PMCID: PMCPMC8409808.
- Yesiladali M, Yazici MGK, Attar E, Kelestimur F. Differentiating Polycystic Ovary Syndrome from Adrenal Disorders. Diagnostics (Basel). 2022;12(9). Epub 2022/09/24. doi: 10.3390/diagnostics12092045. PubMed PMID: 36140452; PubMed Central PMCID: PMCPMC9498167.


