The simplified DUTCH Report has arrived, and current DUTCH Providers are eligible for up to 5 DUTCH Complete or DUTCH Plus test kits for 50% off. Learn more and claim this offer!
The Potential Benefits of “4-Spot Urine Testing” for HPA Axis Assessment
Mark Newman, MS
and Carrie Jones, ND
The Potential Benefits of “4-Spot Urine Testing” for HPA Axis Assessment
by Mark Newman, MS
Written by Carrie Jones, ND and Mark Newman, MS
The health of the entire hypothalamic-pituitary-adrenal (HPA) axis is critically important for maintaining proper health primarily because of cortisol’s role in the body. Cortisol is known as a glucocorticosteroid because of its effects on blood sugar regulation (gluco), it is made in the cortex of the adrenal glands (cortico) and of course, its action as a potent steroid hormone with effects that are far reaching systemically. Released primarily in response to perceived low blood sugar in order to directly stimulate glucuoneogenesis and indirectly, glycogenolysis, cortisol helps to counter inflammation through certain cytokines in the immune system and increases to prepare the body for “fight or flight” as part of the normal, healthy stress response. Like all things, balance is key as high levels can result in inflammatory tissue damage, bone loss, blood sugar dysregulation, memory loss via the hippocampus and insomnia. Low levels may worsen depression and fatigue, result in more inflammation throughout the body, reduce gastric-acid secretion in the stomach and affect proper blood sugar regulation. While there are well-known adrenal gland conditions such as Addison’s and Cushing’s disease, other conditions and symptoms seem to fall on a broadly categorized HPA axis dysfunction spectrum. This is where proper testing comes into play as someone may have low cortisol production causing symptoms such as fatigue and a weakened immune system, however their results are not low enough to diagnose ‘Addison’s Disease’ or warrant cortisol replacement. Alternatively, someone may have high cortisol production causing symptoms like weight gain or insomnia yet it does not fall into the ‘Cushing’s Disease’ category. How is HPA axis dysfunction best tested in order to properly identify problems and treatment when there are many parts to evaluating cortisol through the day such as total production, cortisol clearance, free cortisol values, and the diurnal pattern?
By the middle of the end of the 1950’s there were reasonable solutions to both Addison’s and Cushing ’s diseases.(Contreres, 1986) About the same time, although in relative independence, Hans Selye and others were dramatically increasing our understanding of the stress response. Until the 1980’s lab testing for cortisol was limited primarily to serum cortisol and 24-hour urine testing of free cortisol. In 1981 salivary cortisol testing was first reported. In 2006, Jerjes showed similar patterns of free cortisol in saliva and urine using “spot” urine collections serially throughout the day. (Jerjes, 2005, 2006) They also looked at the metabolites of cortisol. Their research showed that while free cortisol is lower in patients with Chronic Fatigue Syndrome, levels of cortisol metabolites are not. They showed equivalent results when looking at cortisol’s end metabolites in one study. In a separate study, they showed slightly increased metabolites of cortisol in patients with CFS. If free cortisol results are lower and cortisol metabolites are equivalent or higher in patients with CFS, cortisol metabolism/clearance may be up-regulated. Increased clearance of cortisol may ultimately lead to hypocortisol conditions even if the patient’s cortisol production is “normal.” (Jerjes, 2006)
The importance of cortisol clearance – using Anorexia as a case study
Elevations in hormone levels are often assumed to be due to increased hormonal production. In some scenarios, however, elevations may be due decreased metabolism or clearance of a hormone. Such is the case with elevated free cortisol (and DHEA) in anorexia nervosa. In 2012, Oskis published that patients with anorexia nervosa may have “hypersecretion of both cortisol and DHEA.” They concluded this after finding higher levels of both hormones in the saliva of patients with anorexia. However, in 2011 levels of cortisol and DHEA metabolites were published showing dramatically lower levels in patients with anorexia. High levels of free hormone and reduced levels of metabolites implies that the elevation in free hormones may be caused by slow metabolism/clearance of these hormones. (Wassif, 2011)
More than 30 years before the two studies referenced above, Boyar showed that cortisol’s metabolic clearance rate was indeed twice as slow in anorexic patients. (Boyar, 1977) This study also showed that levels of T3 were more than 2.5 times lower in the anorexia patients (more on the role of hypothyroidism later).
As we characterize anorexia as a condition with respect to cortisol, higher levels of free cortisol (which may lead patients to be more at risk for depression and other conditions seen in hypercortisolism) and lower levels of cortisol metabolites are both relevant observations. Knowing both the free and metabolized hormone levels gives insight into situations where clearance/metabolism rates may be abnormal.
Common conditions where cortisol clearance rates may be abnormal:
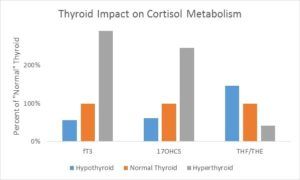
Hypo/Hyperthyroidism – In a 1993 publication, Taniyama published data correlating thyroid status with the excretion rates of cortisol metabolites (also confirmed by Hoshiro, 2006). They measured 17-hydroxycorticosteroid (total of cortisol metabolites, 17-OHCS) levels in urine along with the ratio of cortisol metabolites (THF) to cortisone metabolites (THE). As you can see in the graph below, excretion of cortisol metabolites (17OHCS) generally parallels thyroid status. Patients with higher thyroid levels also show a preference for cortisone metabolites, meaning cortisol tends to spend more time in its inactive form. Conversely, a hypothyroid patient tends to hold on to their cortisol. It is not as readily deactivated to cortisone nor is it cleared as rapidly as 17OHCS cortisol end products. (Taniyama, 1993)
Lab tests available for Cortisol Obesity
Weight gain has been associated with higher cortisol since the proper characterization of Cushing’s Syndrome in the 1930’s by Harvey Cushing. Patients with Cushing’s Disease typically do not show the usual diurnal pattern of cortisol (particularly free cortisol). They exhibit elevated levels throughout the day and are not suppressed by Dexamethazone (Contreras, 1986). These patients also tend to gain weight, especially abdominally. In spite of this correlation, studies have not shown a positive relationship between BMI and free cortisol in either urine or saliva. (Abraham, 2013) Cortisol metabolites, however have been shown to be very strongly correlated to BMI. (Tomlinson, 2008) The above data implies that cortisol production (best approximated by the total of cortisol and cortisone metabolites) is increased in obesity and that cortisol clearance may be up-regulated as well. Adipose tissue also contains higher levels of 11bHSD1, the enzyme responsible for increasing the conversion of the inactive cortisone into cortisol. In many individuals, the activation of this enzyme results in excess cortisol locally resulting in more adipose gain. Measuring the systemic preference for cortisol metabolites versus cortisone metabolites helps to understand the enzymatic activity individually. This further supports the concept that elevated cortisol is a risk factor for abdominal weight gain however it is important to look at cortisol production and clearance, not just free cortisol alone.
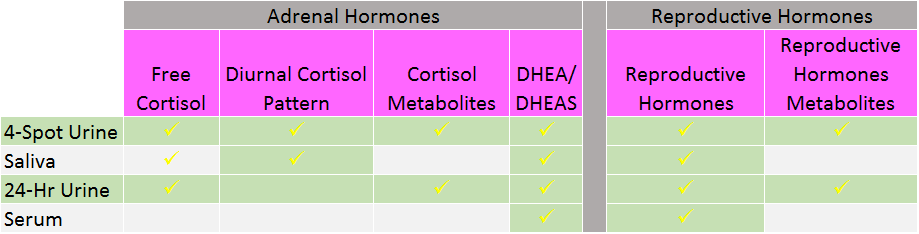
There are several options traditionally used for cortisol testing all with their pros and cons (see box). Serum cortisol requires a blood draw making it difficult to “collect” throughout the day and assess the patient’s cortisol pattern. Due to the nature of the collection, serum results are a mix of bound and unbound cortisol in the blood stream making it extremely difficult to distinguish between the two in one lump sum number. Salivary testing is done by spitting into small tubes at set points throughout the day. While this method does evaluate the daily pattern, it does not include the metabolites of cortisol. Neither serum nor salivary testing has the ability to give insight into the clearance of cortisol. 24-hour urine testing involves the collection of urine in a large container for an entire day. While not entirely pleasant and often inconvenient, this method does report both free and metabolized cortisol however it is unable to graph free cortisol through the day. It is worth noting that some urine labs test “total” cortisol and not “free” cortisol. There is little support in the literature for the measurement of “total” cortisol as measured in urine. The newest model of testing involves a 4-spot dried urine testing for comprehensive hormones whereby patients urinate on small strips of filter paper at set points through the day. The samples dry and are sent back to the lab. This method is able to evaluate the daily free cortisol throughout the day plus report free and metabolized cortisol with a simple collection process that is easily transportable.
Health care as a whole is becoming more integrative, functional and personalized as practitioners are encountering more patients who seem to have increasingly challenging health conditions. Complex cases involving autoimmunity, resistant weight loss, genetic mutations, environmental exposures and nutrient deficiencies have become the norm requiring advanced education, skills, and testing. At the heart of it all sits the hypothalamus-pituitary-adrenal axis trying to manage blood sugar, orchestrate the immune system, and handle the onslaught of stress on a daily basis. Without a proper understanding of the big picture, practitioners may be treating patients for a free cortisol level that only tells half the story. HPA axis dysfunction involves more than just the adrenal glands as the brain, liver and kidneys make-up the rest of the axis from start to finish. Evaluating cortisol production and clearance in addition to the free cortisol levels provides an additional level of information to guide the health care provider in their personalized treatment approach. In order to get this information, providers can either order saliva in combination with 24-urine testing or use a new model of testing – spot urine testing. This new model uses dried urine collections at four specific points in the day. Patients can avoid spitting into a tube or carrying around a 24-hour jug and the dried urine strips are easily mailed. With this method, cortisol testing becomes uniquely comprehensive including both the diurnal cortisol pattern graphed throughout the day plus the metabolites to better approximate overall output of adrenal cortisol production. As an added bonus, the reproductive hormones and their metabolites can be tested as well lending even more insight for the provider and patient.
Ref erences
- Contreras, L. N., S. Hane and J. B. Tyrrell (1986). “Urinary cortisol in the assessment of pituitary-adrenal function: utility of 24-hour and spot determinations.” J Clin Endocrinol Metab 62 (5): 965-969.
- Cook, M. R., C. Graham, R. Kavet, R. G. Stevens, S. Davis and L. Kheifets (2000). “Morning urinary assessment of nocturnal melatonin secretion in older women.” J Pineal Res 28 (1): 41-47.
- Denari, J. H., Z. Farinati, P. R. Casas and A. Oliva (1981). “Determination of ovarian function using first morning urine steroid assays.” Obstet Gynecol 58 (1): 5-9.
- Hoshiro, Y. Ohno, H. Masaki, H. Iwase and N. Aoki (2006) “Comprehensive study of urinary cortisol metabolites in hyperthyroid and hypothyroid patients” Clinical Endocrinology 64 : 37-45
- Iranmanesh, A, Lizarralde, G, Johnson, M, and Veldhuis, J, (1990) “Dynamics of 24-Hour Endogenous Cortisol Secretion and Clearance in Primary Hypothyroidism Assessed before and after Partial Thyroid Hormone Replacement*” Journal of Clinical Endocrinology and Metabolism 70 (1): 155-161
- Jerjes, W. K., A. J. Cleare, S. Wessely, P. J. Wood and N. F. Taylor (2005). “Diurnal patterns of salivary cortisol and cortisone output in chronic fatigue syndrome.” J Affect Disord 87 (2-3): 299-304.
- Jerjes, W. K., T. J. Peters, N. F. Taylor, P. J. Wood, S. Wessely and A. J. Cleare (2006). “Diurnal excretion of urinary cortisol, cortisone, and cortisol metabolites in chronic fatigue syndrome.” J Psychosom Res 60 (2): 145-153.
- C. Vantyghem, A. Ghulam, C. Hober, C. Schoonberg, M. D’Herbomez, A. Racodot, A. Boersma and J. Lefebvre (1998) “Urinary cortisol metabolites in the assessment of peripheral thyroid hormone action: Overt and subclinical hypothyroidism.” J.Endocrinol. Invest . 21:21-225
- Miro, F., J. Coley, M. M. Gani, P. W. Perry, D. Talbot and L. J. Aspinall (2004). “Comparison between creatinine and pregnanediol adjustments in the retrospective analysis of urinary hormone profiles during the human menstrual cycle.” Clin Chem Lab Med 42 (9): 1043-1050.
- Mistry, H. D., N. Eisele, G. Escher, B. Dick, D. Surbek, C. Delles, G. Currie, D. Schlembach, M. G. Mohaupt and C. Gennari-Moser (2015). “Gestation-specific reference intervals for comprehensive spot urinary steroid hormone metabolite analysis in normal singleton pregnancy and 6 weeks postpartum.” Reprod Biol Endocrinol 13 : 101.
- Munro, C. J., G. H. Stabenfeldt, J. R. Cragun, L. A. Addiego, J. W. Overstreet and B. L. Lasley (1991). “Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay.” Clin Chem 37 (6): 838-844.
- Probst-Hensch, N. M., S. A. Ingles, A. T. Diep, R. W. Haile, F. Z. Stanczyk, L. N. Kolonel and B. E. Henderson (1999). “Aromatase and breast cancer susceptibility.” Endocr Relat Cancer 6 (2): 165-173.
- Roos, J., S. Johnson, S. Weddell, E. Godehardt, J. Schiffner, G. Freundl and C. Gnoth (2015). “Monitoring the menstrual cycle: Comparison of urinary and serum reproductive hormones referenced to true ovulation.” Eur J Contracept Reprod Health Care 20 (6): 438-450.
- Taioli, E., A. Im, X. Xu, T. D. Veenstra, G. Ahrendt and S. Garte (2010). “Comparison of estrogens and estrogen metabolites in human breast tissue and urine.” Reprod Biol Endocrinol 8 : 93.
- Taniyama, M, Keiko Honma, K and Ban, Y (1993) “Urinary Cortisol Metabolites in the Assessment of Peripheral Thyroid Hormone Action: Application for Diagnosis of Resistance to Thyroid Hormone” Thyroid 3 (3): 229-233
- Waller, K., S. H. Swan, G. C. Windham, L. Fenster, E. P. Elkin and B. L. Lasley (1998). “Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women.” Am J Epidemiol 147 (11): 1071-1080.
- Oskis, A., C. Loveday, F. Hucklebridge, L. Thorn, and A. Clow (2012) “Diurnal patterns of salivary cortisol and DHEA in adolescent anorexia nervosa” Stress 15 (6): 601-607
- Wassif, S. W., D. M. McLoughlin, R. P. Vincent, S. Conroy, G. F. M. Russell, and N. F. Taylor (2011) “Steroid metabolism and excretion in severe anorexia nervosa: effects of refeeding” The American Journal of Clinical Nutrition 93: 911-917
- Boyar, R. M., L. D. Hellman, H. Roffwarg, J. Katz, B. Zumoff, J. O’Connor, H. L. Bradlow, D. K. Fukushima (1977) “Cortisol secretion and metabolism in anorexia nervosa” New England Journal of Medicine 296 (4): 190-193
- Abraham, S. B., Rubino, N. Sinaii, S. Ramsey, L. K. Nieman (2013) “Cortisol, obesity and the metabolic syndrome: A cross-sectional study of obese subjects and review of the literature” Obesity (Silver Spring) 21 (1): E105-E117
- Tomlinson, J. W., J. Finney, B. A. Hughes, S. V. Hughes, P. M. Stewart (2008) “Reduced Glucocorticoid Production Rate, Decreased 5α-Reductase Activity, and Adipose Tissue Insulin Sensitization After Weight Loss” Diabetes 57: 1536-1543
*This blog post is not intended to diagnose, treat, cure, or prevent any disease. the information provided on this site is not intended as a substitute for advice from your physician or other healthcare professional. Please consult with a healthcare provider before making any changes to your diet, supplements, medications, lifestyle, or if you suspect you might have a health problem.
TAGS
Cortisol
Cortisol Metabolism
HPA Axis
Obesity
Metabolic Health
Women's Health
Men's Health