New Peer-Reviewed Data Shows the Reliability of Urine Hormone Testing to Monitor HRT
Mark Newman, MS
New Peer-Reviewed Data Shows the Reliability of Urine Hormone Testing to Monitor HRT
by Mark Newman, MS
More reasons to trust DUTCH Testing for HRT monitoring
We are always excited to publish data validating our testing, but I have never been more excited to share a Precision Analytical, DUTCH data-driven publication. In the recent edition of Steroids , you will find peer-reviewed data showing the urine response to FDA-approved estradiol gel products.

Five conclusions from our urine hormone test research:
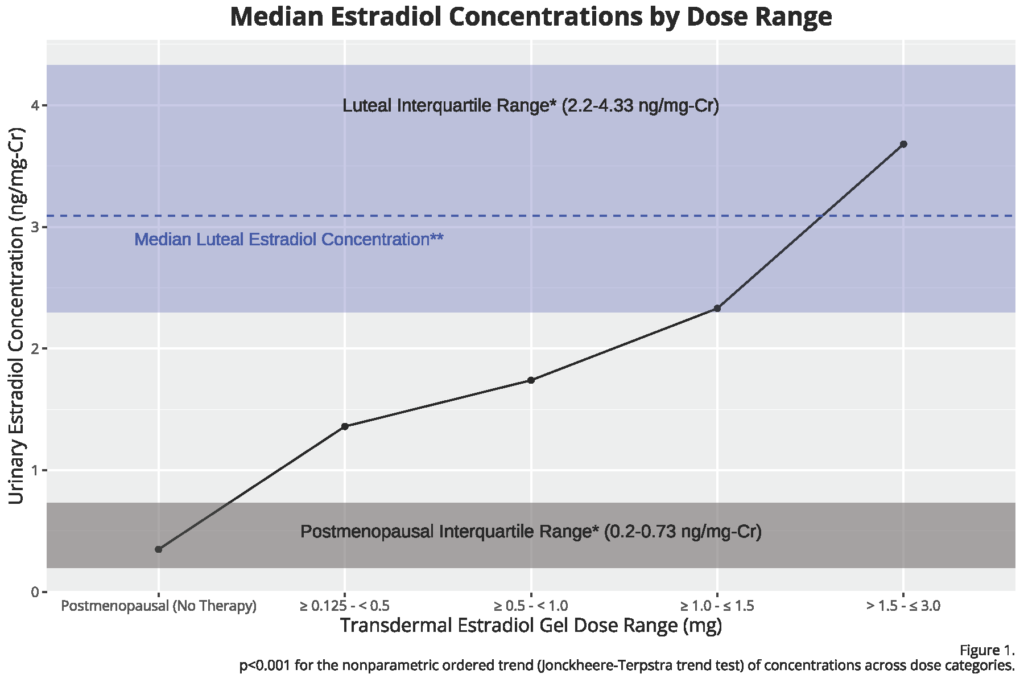
- Urine results scale up in a linear, dose-dependent manner from low doses (0.125-0.4mg) to the highest of doses used clinically.
- The low dose products have been shown in clinical studies to have some, but weak clinical impact (hot flash relief, vaginal atrophy improvement) in most products with the lowest products sometimes failing to show clinical impact. DUTCH results show these products moving estradiol levels from postmenopausal levels to just outside the postmenopausal range. Given the published clinical impact in placebo-controlled studies, urine results are in alignment with the clinical data.
- Urine concentrations in the DUTCH Test may be improved over serum results due to its ability to average out the highs and lows throughout the day (which are quite significant with estrogen gel products).
- Urine concentrations in the DUTCH Test align with reported symptom improvement from clinical studies when compared to salivary values. Published salivary values on similar products are only found in one paper , but the reported values are many times higher than expected given the clinical response of these products.
- Urine metabolite values also scale up with dosing of transdermal estrogen products making DUTCH the optimal test for monitoring overall estrogen exposure as well as metabolite patterns.
Settling the HRT Monitoring Controversy
There has long been debate about whether or not urine testing can be used to monitor transdermal estrogen creams and gels. We look forward to a day when outcome studies are completed with comprehensive lab testing to further clarify the relationship between lab values and specific clinical goals. Read through our other peer-reviewed published research papers to explore the available data in more detail.
You can also walk through this latest research with me on our YouTube channel . In this video, I explain the data to help providers sift through the noise and get to the bottom of this heavily debated controversy.
TAGS
Functional Laboratory Testing: General
Hormone Replacement Therapy (HRT)
Laboratory Testing: In-Depth Look at DUTCH Urine Testing
Menopause