Metabolic Factors in PCOS
Tim Hyatt, ND
Metabolic Factors in PCOS
by Tim Hyatt, ND
One of the possible etiologies of polycystic ovary syndrome (PCOS) is metabolic dysregulation and thus it may be more appropriately called “metabolic reproductive syndrome”. This article will review some aspects of the current clinical and scientific understanding of PCOS and consider how metabolic factors affect the clinical picture of PCOS.
In an article titled Diagnoses, Syndromes, and Diseases: A Knowledge Representation Problem, Calvo et al describe a syndrome as follows, “a syndrome is a recognizable complex of symptoms and physical findings which indicate a specific condition for which a direct cause is not necessarily understood.” PCOS is a great example of a syndrome because there is a cluster of signs and symptoms, but the scientific and clinical communities have been unable to isolate a specific cause. Yet, this condition affects a great number of people, and many providers still don’t understand a highly effective approach to diagnosis and treatment.
Diagnostic Criteria of PCOS
The scientific and medical communities consider PCOS to be an ovarian disorder centered around reproductive manifestations. Diagnostic criteria have been established multiple times by the NIH in 1990, the Rotterdam Criteria in 2003, and the Androgen Excess Society in 2006. Each organization uses the clinical features of hyperandrogenism, oligo or amenorrhea, and the presence of polycystic ovaries, but there is no consensus on the combination of those clinical features used to establish this diagnosis.
Some of the barriers to agreement pertain to the observation of polycystic ovaries, which is influenced by the low availability of ultrasound equipment in medical offices, the lack of expertise in imaging techniques, and the lack of consensus for diagnosis and treatment within the scientific community.
Urine testing is not diagnostic for PCOS. However, high levels of androgen metabolites, including testosterone, 5a-DHT, 5a-androstenediol, and androsterone may be observed in patients with suspected PCOS. Elevated levels of alpha metabolites are the norm in patients who have previously been diagnosed with PCOS. Oligo or amenorrhea are easily observed by documenting the timing of cycles, and ultrasound of ovaries is recommended when hyperandrogenism and cycle abnormalities are found.

Clinical Manifestations
Manifestations of PCOS are metabolic dysregulation—including overweight and obesity, insulin resistance, increased lipids and elevated cardiovascular risks, diabetes, beta cell dysfunction, hair loss on the scalp, hirsutism, acne, nonalcoholic steatohepatitis and sleep apnea, mood disorders, and binge eating.
Incidence and Prevalence of PCOS
PCOS affects 5-13% of women of reproductive age, depending on the diagnostic criteria used. Obesity or overweight affects 38-88% of women with PCOS, and insulin resistance (IR) is also common among patients with PCOS, with a prevalence of 35%-80%. Other conditions associated with an increased prevalence of PCOS include oligo ovulatory infertility, types I, II, and/or gestational diabetes, premature adrenarche, a heritable component in first-degree relatives with PCOS, and the use of valproic acid.
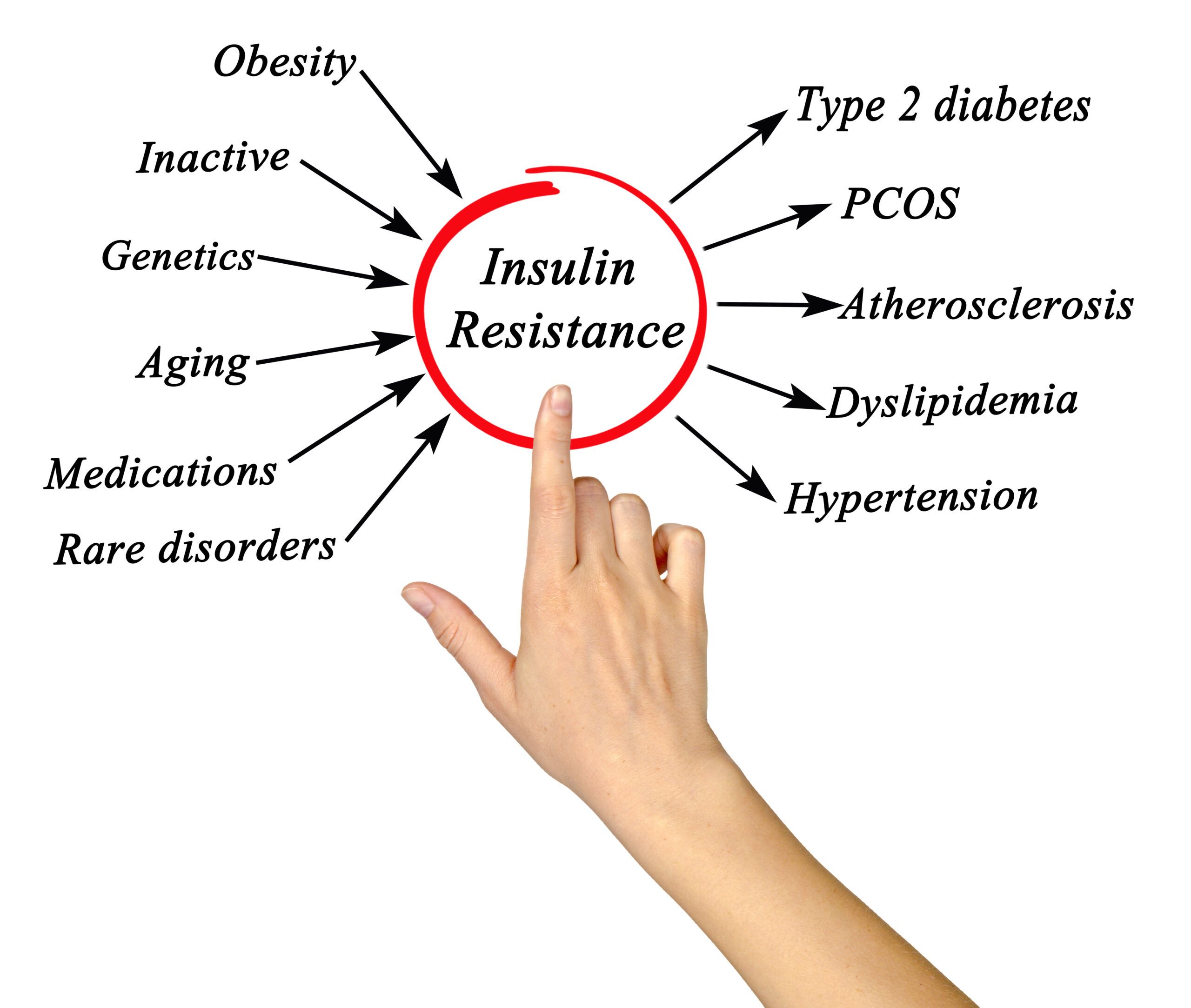
Possible Etiologies
The etiology of PCOS is unknown, and there are many factors that have a strong association with its prevalence in the US and around the world. Theories about its etiology and pathogenesis include:
- Alteration in gonadotropin secretion
- Ovarian and adrenal dysfunction leading to hyperandrogenism
- Hyperinsulinemia, disordered insulin action, and obesity
- Genetic predisposition
HPO Axis and Insulin Resistance
When evaluating the possible etiologies of PCOS, two sets of characteristics emerge as interdependent processes. The first set is the hypothalamic pituitary ovarian axis (HPO), and the second is the hyperandrogenism combined with disordered insulin response.
In the HPO axis, ovarian output is entirely dependent on gonadotropin signaling. Once signaling is initiated and the ovaries respond to the onset of menarche, the gonadotropins and ovaries operate as a single unit. This continues unless disrupted by pathological changes or exogenous influences until the onset of menopause. If the gonadotropin signaling is inadequate, development of the follicle and corpus luteum is altered. This ongoing cycle dysregulation involves elevated androgens and abnormal insulin response.
Women with PCOS frequently have insulin resistance and impaired insulin response, hyperandrogenism, HPO dysfunction, and ovarian cysts. Hyperandrogenism and insulin dysregulation are processes linked together in a self-perpetuating cycle. As each of them rise, the positive and negative feedback loops of the gonadotropins and the ovaries are impaired, and the menstrual cycle is disrupted because insulin causes theca cells and granulosa cells to generate and release androgens.
The ovaries seem to have the biggest influence in hyperandrogenism and PCOS, but androgen production is often increased in the adrenal gland due to genetic predisposition, disrupted signaling, and hyperinsulinemia. The altered signaling and hyperinsulinemia tend to increase hyperandrogenism unless treatments are used to disrupt the process.
In a small study, Arroyo et al showed that thin and obese patients diagnosed with PCOS had abnormal postprandial and fasting insulin response when compared with controls. Many women with PCOS have fasting and or meal-challenged hyperinsulinemia. Consequently, increased levels of insulin results in low circulating levels of sex hormone-binding globulin SHBG) and higher free testosterone levels. Some have suggested that low circulating SHBG levels may be a good marker for women with PCOS because elevated androgens can also suppress SHBG.
Kursad et al noted that, “not all signaling pathways and insulin-responsive tissues are equally affected, and some effects other than the metabolic actions of insulin are overexpressed. Ovaries and the adrenal glands are two examples of tissues remaining sensitive to insulin actions where insulin may contribute to increased androgen.”
Insulin resistance and hyperinsulinemia were also found to affect endometrial physiology as well as the ovaries. Lee et al found that the function of insulin receptors, insulin receptor substrate proteins, and glucose transporters are dysregulated in the endometrium of women with PCOS.
Another factor to consider is that hepatic insulin resistance (insulin resistance in liver cells resulting in impaired glycogen synthesis) fails to suppress glucose production, enhances lipogenesis, and increases the synthesis of proteins such as CRP. This is only seen in obese women with PCOS when compared to “healthy” women of equivalent body weight. Obesity and PCOS have a compounding negative effect on endogenous glucose production, which may play a role in the etiology of glucose intolerance.
In addition to androgen production in the ovaries, the adrenal glands produce roughly half of the androgens in the body; therefore, DHEA and androsterone will be increased in patients with adrenal PCOS. There appears to be a genetic component to adrenal PCOS and Khasar-Miller showed that familial clustering of elevated DHEA-S levels in PCOS families in both female and male relatives mark a heritable component to this trait.
Other factors to consider are genetics, basal metabolic rate (BMR), and premature adrenarche (PA). For patients who are insulin-resistant with PCOS, their BMR is around 1,116 calories compared to the average BMR of 1,868 calories in women without PCOS. This may be the most important factor for the lack of success in weight reduction, insulin resistance, and androgen output for patients with PCOS.
Premature adrenarche is associated with hyperinsulinemia and childhood overweight. In girls with premature adrenarche, anti-mullerian hormone (AMH) was elevated when compared with healthy controls with no history of PA. Additionally, this study found that maternal history of PCOS was related to even higher levels of AMH, often correlated with higher risk for PCOS.
Conclusion
PCOS is an incredibly complex syndrome with many clinical factors that are interconnected. Researchers are hard at work trying to find pharmacologic therapies that are helpful for symptom management, but from a functional standpoint, providers must look at this condition and identify factors they can modify to help patients move forward in their quest to be healthy. Arguably, the metabolic features of PCOS are tightly linked to reproductive processes and function, and clinical interventions should be directed toward those features for effective treatment.
References
- Calvo , Franz, MD, 1 Karras , Bryant T, MD, 1,2 Phillips , Richard , MD, 1 Kimball , Ann Marie MD, 1,2 and Wolf , Fred, Diagnoses, Syndromes, and Diseases: A Knowledge Representation Problem , AMIA Annu Symp Proc. 2003; 2003: 802.
- Richard S. Legro, M.D. Evaluation and Treatment of Polycystic Ovary Syndrome , Department of Obstetrics and Gynecology, Penn State College of Medicine, 500 University Drive, Hershey, PA, USA. Updated: January 11, 2017.
- Ehrmann DA. Polycystic ovary syndrome . The New England journal of medicine 2005; 352:1223-1236.
- Azziz, Ricardo MD, MBA, MPH , Barbieri, Robert L MD, Martin, Kathryn A MD , Epidemiology, phenotype, and genetics of the polycystic ovary syndrome in adults Literature review current through: Jul 2023.
- Barbieri, Robert L , MD , Ehrmann, David A, MD , Crowley, William F Jr, MD , Martin, Kathryn A , MD , Diagnosis of polycystic ovary syndrome in adults Literature review current through: Jul 2023.
- Legro RS, Myers ER, Barnhart HX, Carson SA, Diamond MP, Carr BR, Schlaff WD, Coutifaris C, McGovern PG, Cataldo NA, Steinkampf MP, Nestler JE, Gosman G, Guidice LC, Leppert PC. The Pregnancy in Polycystic Ovary Syndrome study: baseline characteristics of the randomized cohort including racial effects. Fertility and sterility 2006; 86:914-933.
- Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, Christman GM, Huang H, Yan Q, Alvero R, Haisenleder DJ, Barnhart KT, Bates GW, Usadi R, Lucidi S, Baker V, Trussell JC, Krawetz SA, Snyder P, Ohl D, Santoro N, Eisenberg E, Zhang H, Network NRM. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome . The New England journal of medicine 2014; 371:119-129.
- Barber TM, McCarthy MI , Wass J A H, Franks S, Obesity and polycystic ovary syndrome 2006 , Clin Endocrinol (Oxf) Aug;65(2):137-45.
- Chantal Anifa Amisi , Markers of Insulin Resistance in Polycystic Ovary Syndrome women: An update, World J Diabetes. 2022 Mar 15; 13(3): 129–149. Published online 2022 Mar 15. doi: 4239/wjd. v13.i3.129 .
- Ehrmann DA. Polycystic ovary syndrome. The New England journal of medicine 2005; 352:1223-1236.
- Rosenfield Robert L., Ehrmann , David A., The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited . Published online 2016 Jul 26. doi: 1210/er.2015-1104 .
- Arroyo A, Laughlin GA, Morales AJ, Yen SS. J, Inappropriate gonadotropin secretion in polycystic ovary syndrome: influence of adiposity. Clin Endocrinol Metab. 1997 Nov;82(11):3728-33. doi: 10.1210/jcem.82.11.4377.PMID:
- Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease . The Journal of clinical endocrinology and metabolism 1983; 57:356-359.
- Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS., Clore JN, Blackard WG. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome . The Journal of clinical endocrinology and metabolism 1991; 72:83-89.
- Cumming DC, Wall SR. Non-sex hormone-binding globulin-bound testosterone as a marker for hyperandrogenism . The Journal of clinical endocrinology and metabolism 1985; 61:873-876.
- Kursad Unluhizarci , Zuleyha Karaca , and Fahrettin Kelestimur , Role of insulin and insulin resistance in androgen excess disorders , World J Diabetes. 2021 May 15; 12(5): 616–629, Published online 2021 May 15. doi: 4239/wjd. v12.i5.616 .
- Oróstica L, Rosas C, Plaza-Parrochia F, Astorga I, Gabler F, García V, Romero C, Vega M. Altered Steroid Metabolism and Insulin Signaling in PCOS Endometria: Impact in Tissue Function . Curr Pharm Des. 2016; 22:5614–5624.
- Lee MH, Yoon JA, Kim HR, Kim YS, Lyu SW, Lee BS, Song H, Choi DH. Hyperandrogenic Milieu Dysregulates the Expression of Insulin Signaling Factors and Glucose Transporters in the Endometrium of Patients with Polycystic Ovary Syndrome . Reprod Sci. 2020; 27:1637–1647.
- RezaMeshkani a , Khosrow Adeli b Hepatic insulin resistance, metabolic syndrome and cardiovascular disease , Clinical Biochemistry , Volume 42, Issues 13–14 , September 2009, Pages 1331-1346.
- Gholinezhad M, Gholsorkhtabaramiri M, Esmaeilzadeh S, Ghanbarpour A. Insulin Resistance and Adverse Metabolic Profile in Overweight/Obese and Normal Weight of Young Women with Polycystic Ovary Syndrome. Caspian J Intern Med (2018) 9(3):260. doi: 10.22088/cjim.9.3.260.
- Farese RV Jr., Zechner R, Newgard CB, Walther TC. The Problem of Establishing Relationships Between Hepatic Steatosis and Hepatic Insulin Resistance . Cell Metab (2012) 15(5):570–3. doi: 10.1016/j.cmet.2012.03.004.
- Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS . Fertility and sterility 2001; 75:53-58.
- Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proceedings of the National Academy of Sciences of the United States of America 1998; 95:14956-14960.
- Legro RS, Kunselman AR, Demers L, Wang SC, Bentley-Lewis R, Dunaif A. Elevated dehydroepiandrosterone sulfate levels as the reproductive phenotype in the brothers of women with polycystic ovary syndrome . The Journal of clinical endocrinology and metabolism 2002; 87:2134-2138.
- Quinkler Marcus, Binayak Sinha , Jeremy W Tomlinson , Iwona J Bujalska , Paul M Stewart , Wiebke Arlt , Androgen generation in adipose tissue in women with simple obesity--a site-specific role for 17beta-hydroxysteroid dehydrogenase type 5, J Endocrinol. 2004 Nov;183(2):331-42.
- Vink JM,
- Mykhalchenko K, Lizneva D, Trofimova T, et al.
- Georgopoulos NA, Saltamavros AD, Vervita V, et al. Basal metabolic rate is decreased in women with polycystic ovary syndrome and biochemical hyperandrogenemia and is associated with insulin resistance. Fertility and Sterility. 2009;92(1):250-255. doi:10.1016/j. fertnstert.2008.04.067.
- Jani Liimatta,Pauliina Utriainen, Tomi Laitinen, Raimo Voutilainen, Jarmo Jääskeläinen; Cardiometabolic Risk Profile Among Young Adult Females With a History of Premature Adrenarche , Journal of the Endocrine Society , Volume 3, Issue 10, October 2019, Pages 1771–1783, https://doi.org/10.1210/js.2019-00193 , Published: 15 July 2019.
- AlexandraEfthymiadou MD, PhD, Maria Bogiatzidou MD, Dimitra Kritikou PhD, Dionisios Chrysis MD, PhD Anti-Müllerian Hormone in Girls with Premature Adrenarche: The Impact of Polycystic Ovary Syndrome History in their Mothers The Journal of Pediatrics , Volume 205 , February 2019, Pages 190-194. Endocr Rev. 2016 Oct; 37(5): 467–520.
- Yildiz BO, Knochenhauer ES, Azziz R. Impact of obesity on the risk for polycystic ovary syndrome . The Journal of clinical endocrinology and metabolism 2008; 93:162-168.
- Teede HJ, Joham AE, Paul E, Moran LJ, Loxton D, Jolley D, Lombard C. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women . Obesity (Silver Spring) 2013; 21:1526-1532
- Srikanthan P, Korenman S, Davis S. Polycystic ovarian syndrome: the next cardiovascular dilemma in women? Endocrinol Metab Clin North Am. 2006 Sep;35(3):611-31, x. doi: 10.1016/j.ecl.2006.05.001.PMID: 16959589
- Barber TM, Why are women with polycystic ovary syndrome obese? British Medical Bulletin , Volume 143, Issue 1, September 2022, Pages 4–15, https://doi.org/10.1093/bmb/ldac007 , Published: 12 March 2022.
- Barber TM, Franks S,(2010) Genetic basis of polycystic ovary syndrome , Expert Review of Endocrinology & Metabolism, 5:4, 549-561, DOI: 1586/eem.10.32 .
- Saadia Z. Follicle Stimulating Hormone (LH: FSH) Ratio in Polycystic Ovary Syndrome (PCOS) - Obese vs. Non- Obese Women . Med Arch. 2020 Aug;74(4):289-293. doi: 10.5455/medarh.2020.74.289-293. PMID: 33041447.
- Schneyer AL, Fujiwara T, Fox J, Welt CK, Adams J, Messerlian GM, Taylor AE. Dynamic changes in the intrafollicular inhibin/activin/follistatin axis during human follicular development: relationship to circulating hormone concentrations. The Journal of clinical endocrinology and metabolism 2000; 85:3319-3330.
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome . Diabetes 1989; 38:1165-1174.
- Dunaif A, Finegood DT. Beta-cell dysfunction independent of obesity and glucose intolerance in the polycystic ovary syndrome . The Journal of clinical endocrinology and metabolism 1996; 81:942-947.
- Burt Solorzano CM, McCartney CR, Blank SK, et al. Hyperandrogenaemia in adolescent girls: Origins of abnormal gonadotropin-releasing hormone secretion.
- Haigang Ding 1,2 Juan Zhang 1,2 Feng Zhang 1,2 Songou Zhang 3 Xiaozhen Chen 3 Wenqing Liang 4* Qiong Xie 5* Resistance to the Insulin and Elevated Level of Androgen: A Major Cause of Polycystic Ovary Syndrome , Frontiers in Endocrinology., 20 October 2021Sec. Reproduction Volume 12 – 2021.
- Garg D, Tal R. Inositol Treatment and ART Outcomes in Women With PCOS . Int J Endocrinol (2016) 2016:1–9. doi: 10.1155/2016/1979654.
TAGS
Women's Health
Premenopausal Women
Androgens (Testosterone/DHEA)
Metabolic Health
Insulin Resistance
Polycystic Ovary Syndrome (PCOS)
Obesity
Hair Loss
Inflammation