Menopausal HRT and Cardiovascular Disease Risk
Hilary Miller, ND
and Kelly Ruef, ND
Menopausal HRT and Cardiovascular Disease Risk
by Clinical Consulting Team
This post was co-written by our clinical experts Hilary Miller, ND, and Kelly Ruef, ND.
Heart disease is the leading cause of death in the United States and globally. During menopause when estrogen levels drop, a woman’s risk of cardiovascular disease increases. This is because estrogen provides a protective effect against heart disease in women. In fact, estrogen has been shown to decrease blood pressure, improve cholesterol, and protect against atherosclerosis.
If estrogen plays an important role in supporting cardiovascular health in women, why isn’t menopausal hormone replacement therapy (MHRT) an option for all women? It turns out that some studies have shown that MHRT can increase cardiovascular disease (CVD) risk. This increased risk, however, is likely influenced by the formulation and route of administration of the MHRT prescribed, a woman’s health history, and age at which MHRT is initiated.

Menopausal hormone changes are associated with increased cardiovascular disease risk
Estrogen provides a protective effect against heart disease in women. It lowers blood pressure by increasing blood flow and dilating small arteries, improves cholesterol by lowering LDL and increasing HDL, protects against atherosclerosis, and even helps prevent low vitamin D levels and osteoporosis, two factors that are associated with an increase in cardiac events. It’s no wonder then, that during menopause when estrogen levels drop, a woman’s risk of cardiovascular disease increases. Remember that menopause is the cessation of ovarian hormone cycles and fertility in women that occurs anywhere from age 40-58, with the average age being 51.
What is Menopausal HRT?
Menopausal HRT is a collection of prescription hormone receptor agonists (synthetic or bioidentical hormones) used in menopause to reduce or eliminate symptoms of menopause. It generally refers to the prescription of estrogens and progestogens, although other hormones are sometimes used in post-menopausal women.
MHRT is not currently approved for the prevention or treatment of cardiovascular disease, but it is approved for the following:
- Moderate to severe vasomotor symptoms (VMS)
- Moderate to severe vulvovaginal symptoms: vaginal atrophy (VVA) and dyspareunia
- Prevention of osteoporosis in postmenopausal women
- Hypoestrogenism from hypogonadism, surgical menopause (bilateral oophorectomy), or premature ovarian insufficiency (POI)
It is important to note that there are many forms of estrogens available: conjugated equine estrogens (CEEs), synthetic conjugated estrogens, esterified estrogens, synthetic ethinyl estradiol, and bioidentical estradiol (17β-estradiol; often simply referred to as E2).
Likewise, there are many forms of progestogens available. Progestogen is an umbrella term that includes synthetic and bioidentical forms. Progestins are synthetic progestogens. Progesterone is a bioidentical progestogen. Progestogens are combined with estrogens in women with a uterus to reduce the risk of endometrial hyperplasia that occurs with estrogen.
There are various contraindications to MHRT. The cardiovascular-related contraindications include history of:
- Venous thromboembolism (VTE), as seen with deep vein thrombosis (DVT) or pulmonary embolism (PE)
- Stroke
- Myocardial infarction (heart attack)
- Coronary heart disease (CHD)
- Blood clotting disorders
It is interesting to note that there is some evidence that stroke, heart attack, and clotting risk is improved with estrogen therapy depending on the formulation, route of administration, and health of the patient.
The Women’s Health Initiative
The most prominent study on HRT is the Women’s Health Initiative (WHI). It was a randomized controlled trial (RCT) looking at women aged 50-79. This study used only oral conjugated equine estrogens (CEEs) and oral synthetic progestin, medroxyprogesterone acetate (MPA). CEEs alone were used in women without a uterus and CEEs + MPA in women with a uterus. The study DID NOT use bioidentical estradiol (E2) or bioidentical progesterone.
The initial interpretation of the WHI hurt the public perception of MHRT, as initial negative results were heavily publicized. However, subsequent careful analysis demonstrated the WHI had many positive findings, especially for women aged 50-59 (the most common type of MHRT patient), and all cardiovascular risks were considered rare . For example, when combining the data from both oral CEE + MPA (average use 5.6 years) and oral CEE alone (average 7.2 years) groups after a median of 18 years follow-up, the WHI outcomes for women aged 50-59 found no increased CVD mortality, reduced coronary heart disease (CHD), and a reduction in all-cause mortality!

Women aged 50-59 taking oral CEE had an increased incidence of VTE, however, and women taking oral CEE + MPA additionally had an increased incidence coronary heart disease (CHD) and stroke. As stated earlier, although these risks were identified, the risk increases remained “rare” at <10/10,000 women per year. Despite these incidents remaining in the “rare” category, subjects taking oral MPA, the synthetic progestin, had double the risk of VTE and significantly greater CVD risk than those who did not take MPA. Subsequently, oral MPA has been linked to increased thrombogenesis (increased clotting factors) where oral bioidentical progesterone has not.
It is now over 20 years since the WHI, and continued follow-up and data analysis found that the MHRT used in the study has more health benefits than risks when initiating in women aged 50-59 or within 10 years of menopause. Combining the data from the WHI and other studies, it has been found that the oral route for estrogens and synthetic progestins are thrombogenic, and age as well as baseline health should be considered. Therefore, most major associations conclude that the rare risks identified may be mitigated by appropriate CVD screening and using safer formulations and routes of administration (e.g., transdermal E2 instead of oral CEE and bioidentical progesterone instead of MPA, which will be discussed next).
Graph:
Benefits and Risks of CEE alone or in Combination with MPA
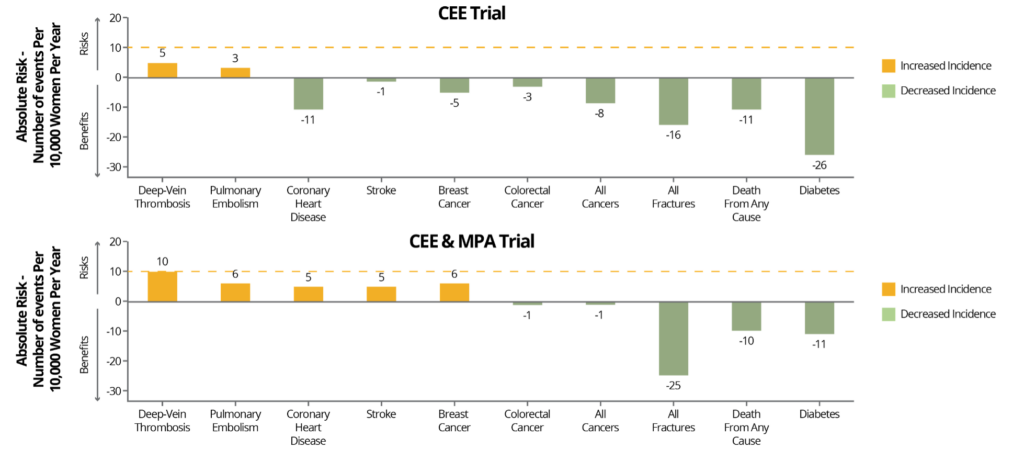
NAMS. Menopause . 2022;29(7):767-794.
Benefits and risks of the two hormone therapy formulations, conjugated equine estrogens (CEE) alone or in combination with medroxyprogesterone acetate (MPA), evaluated in the Women’s Health Initiative for women aged 50-59 years. Risks and benefits are expressed as the difference in number of events (number of hormone therapy group minus the number in the placebo group) per 10,000 women per year, with <10 per 10,000 per year representing a rare event (dashed red line). Adapted from Manson JE, et al.
The formulation and route of administration of MHRT affects CVD risk
Oral estrogen is the most used route of administration (ROA) when it comes to estrogen therapy. It is safe for most patients who are healthy, but transdermal (TD) estrogen is even safer. This is because oral estrogen therapy goes through extensive first-pass metabolism which increases liver clotting factor synthesis. Increases in liver production of blood clotting factors is thought to be the driver of increased VTE and thrombosis risk. Due to these impacts on clotting factors, oral estrogen carries increased risk over TD estrogen therapy for VTE, stroke, clotting, and thrombosis related CVD risks. Keep in mind that in most women, the increased clotting factors is too small to impact their risk but may add on to other risk factors such as genetics and smoking status.
TD estrogen therapy has not demonstrated increased VTE and stroke risk, likely due to bypassing liver metabolism, however, TD estrogen therapy has not been as thoroughly studied as oral estrogens.
Calculating a patient’s 10-year CVD risk is simple and tools can be found online from the American College of Cardiology (ACC). High cholesterol, high blood pressure, diabetes, and being a smoker will increase the patient’s risk. Luckily these factors are modifiable. The benefits of MHRT may be enhanced by improving CVD lifestyle risk factors.
Due to its effect VTE and stroke risk, oral estrogen should not be considered in women with a moderate to high calculated 10-year CVD risk (5 to 10%). Transdermal E2 is a safer alternative to oral E2 in cases where CVD risk is not low, but estrogen therapy is indicated. Keep in mind that caution is advised with patients at high stroke or VTE risk for all estrogen therapy.
Table:
Evaluating CVD Risk in Females Contemplating MHRT
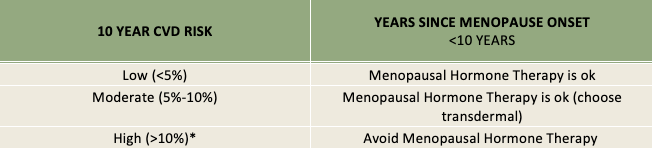
CVD risk is calculated by American College of Cardiology / American Heart Association Cardiovascular Risk Calculator. Methods to calculate risk and risk stratification vary among countries. High risk includes known myocardial infarction (MI), stroke, peripheral artery disease, etc.
The formulation of estrogen and progestogen therapy also affect CVD risk. Oral E2 or TD E2 and oral micronized progesterone (OMP) show even less VTE risk compared to oral CEEs + MPA. Overall, patients with low or normal VTE risk need not avoid oral E2 + OMP if that is their preference, however, patients with an increased risk for VTE or stroke or those over 60 for whom estrogen therapy is indicated should consider using TD E2.
The age at which MHRT is initiated affects CVD risk
It is well-known that MHRT benefits are maximized, and risks minimized, when MHRT is initiated before the age of 60 or within 10 years of menopause. In women older than 59 who initiate HRT for the first time, the benefits, measured over aggregate (group) data, are not as strong. The WHI found a null effect on CHD and all-cause mortality, whereas starting earlier improved these outcomes. The WHI also found an increased risk of stroke and VTE compared to women who initiated HRT at a younger age.
Some have interpreted the reduced benefit profile of women who initiate HRT later to mean that women should stop HRT at a certain age, but the North American Menopause Society (NAMS) and other associations emphasize that these two groups are different. Starting young and staying on HRT is not necessarily the same thing as starting HRT after the age of 59. In any case, the appropriateness of HRT should always be individualized, regardless of age.
Conclusion
As heart disease is the leading cause of death in the United States and globally, careful consideration of a patient’s prior health history and age, and formulation and ROA of estrogens and progestogens should be given prior to writing an MHRT prescription. Although MHRT, especially bioidentical transdermal estrogen therapy, may lead to improvements in cardiovascular health for some women, it could be detrimental to others. Therefore, because every woman who is going through or has already gone through menopause is unique, MHRT should always be individualized.
Become a Provider to learn more about MHRT and gain access to expert clinical support and education, comprehensive patient reports, and validated and peer-reviewed research.
References:
- RenouxC, et al. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340(jun03 4):c2519-c2519.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29(7):767-794.
- Van ErpecumKJ, et al. Different hepatobiliary effects of oral and transdermal estradiol in postmenopausal women. Gastroenterology. 1991;100(2):482-488.
- Walsh BW, et al. Effects of Postmenopausal Estrogen Replacement on the Concentrations and Metabolism of Plasma Lipoproteins. N Engl J Med. 1991;325(17):1196-1204.
- GoldštajnMŠ, et al. Effects of transdermal versus oral hormone replacement therapy in postmenopause: a systematic review. Arch Gynecol 2022;307(6):1727-1745.
- Abou-Ismail MY, et al. Estrogen and thrombosis: A bench to bedside review. Thromb2020;192:40-51.
- BińkowskaM, et al. Position Statement by Experts of the Polish Menopause and Andropause Society on menopausal hormone therapy with an oral combination drug containing oestradiol 1 mg and progesterone 100 mg. Menopausal Review. 2021;20(3):113-115.
- Casper R. Clinical manifestations and diagnosis of menopause. In: Post T, editor. UpToDate. Waltham, MA 2023.
TAGS
Women's Health
Hormone Replacement Therapy (HRT)
Cardiovascular Disease
Postmenopausal Women
Menopause
Estrogen and Progesterone