Estriol Supplementation and Metabolites – A Caveat
Mark Newman, MS
Estriol Supplementation and Metabolites – A Caveat
by Mark Newman, MS
I want to discuss a complicated scenario with you. If you give estriol, please pay attention. If you don’t use estriol in your practice, feel free to forward this to those who do!

Bottom Line: When you take estriol (especially if it is oral/sublingual) some will get converted to 16-OH-E1, E3 will NOT increase 2-OH-E1 or 4-OH-E1.If you’regiving E3 and evaluating the ratios (like the 2/16 ratio), don’t expect these to improve much with things like DIM/I3C (which increase 2-OH estrogens). If the 16-OH-E1 is NOT coming from the E3, DIM/I3C will usually lower 16-OH-E1 (along with E1 and E2).
The goal of this communication is to prevent this type of simple mistake:
- Postmenopausal patient on estriol (only) tests and has low levels for every hormone except estriol (because they are taking it) and 16-OH-E1 (because that is the only hormone estriol can turn into)
- The provider sees a low 2-OH-E1 and a relatively high 16-OH-E1 and gives DIM
- The DIM lowers the E1 and E2 (which are already low) significantly, but doesn’t lower 16-OH-E1 or estriol.
- The DIM increases 2-OH-E1 somewhat (not a lot because there just isn’t much more E1 and E2 that can head that way and estriol cannot be turned into 2-OH-E1)
- The frustrated provider and patient don’t understand why their estrogen ratios look “bad” even though they are taking DIM to improve it and the patient may feel worse with even lower E1 and E2 levels.
When we look at the estrogen metabolites, one of the things we look at is the ratio between the 2-OH, 4-OH, and 16-OH estrogens. We must remember Estriol is 16-OH-E2. There can be some conversion directly from 16-OH-E1 toE3 but also some back the other direction from E3 to 16-OH-E1 (16-OH-E2→ 16-OH-E1). We don’t have an arrow on our report showing that, but it can happen. So if you look at this example, it seems pretty clear that the metabolism is fairly 16-hydroxylation dominant (79.9% of the metabolites are 16-OH-E1 and very little 2-OH-E1). It is generally not considered a good thing that there is more 4-OH-E1 than 2-OH-E1, and I think it is wise to address that, but there is an issue that should be raised here.
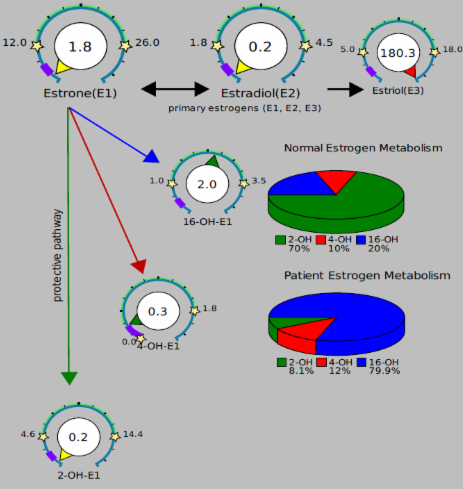
The 2.0 16-OH-E1 is likely coming directly from E3, not E1 or E2. Specifically as it relates to oral E3, what IF this was coming from gut metabolism and it looked like this (all in the gut):
E3 → 16-OH-E1 → conjugation to 16-OH-E1-glucuronide (the form we measure) → circulates as the conjugated form → urine
As opposed to this:
E3 → 16-OH-E1 → circulates as the free form of 16-OH-E1 → conjugation (liver) → circulates briefly as the conjugated form → urine
What’s my point? These two scenarios look the same on the lab test, but only the second one allows 16-OH-E1 to do anything at the tissue level. It is a particularly potent metabolite (more reactive with the estrogen receptor than other metabolites although only about 20% as active as E2). I think it is likely that the first scenario is a major contributor to what’s seen in urine IF taking oral/sublingual E3.
What would I do? I would somewhat ignore the 16-OH-E1 in this case and focus more so on the ratio between 2-OH and 4-OH. In this case, it is still poor. If 2-OH-E1 was 1.5 and 4-OH-E1 was 0.2, the ratio would be slanted heavily towards 2-OH-E1 (versus 4-OH-E1) and that would be good. But, it would not be slanted towards the 2-OH when compared to 16-OH-E1 and mountains of DIM/I3C is not going to change this much. If the 16-OH-E1 is being made in the gut, we need to be careful with our interpretation.
Things like DIM/I3C push E1 and E2 down the 2-OH pathway. This tends to lower 16-OH-E1 because it lowers the amount of estrogen going down the 16-OH pathway. If you give E3, that is already down the 16-OH pathway (since it IS16-OH-E2).
Cheers!
TAGS
Laboratory Testing: In-Depth Look at DUTCH Urine Testing
Estrogen Detoxification
Estrogen and Progesterone
Functional Laboratory Testing: General
Women's Health
Premenopausal Women
Postmenopausal Women
Hormone Replacement Therapy (HRT)
Menopause