A Clinical Nail in the Coffin of the Saliva Testing Model for Transdermal Testosterone Monitoring
by Mark Newman, MS
Download a copy of this article here.
What happens when women take 5-20mg of transdermal testosterone (TD T)? The answer contributes to a fundamental question about TD T laboratory monitoring. In this blog, I will show you that there are a decent number of studies that answer this question with a consistent message. I am also sharing, for the first time, data from women who have done the DUTCH test while on TD T.
It is stating the obvious to say that female patients do not fare well if given typical male doses of testosterone. If, for example, I proposed weekly T cypionate injections of > 200mg or Q 3-month 750-1000mg pellet insertions for your testosterone deficient female patients, you would rightly object. Results would, of course, be disastrous, unless the woman is desiring facial/body hair and acne or is partaking in sex reassignment. Additionally, their voices would deepen, among other high androgen symptoms that you might expect.
Now let’s use the above examples as a backdrop to educate ourselves regarding the controversy surrounding TD T therapy. If I determine the TD T dose that is effective in treating hypogonadal men and give that same dose to testosterone deficient female patients, what will happen? As with T injections, potential adverse outcomes along with androgen excess symptoms may occur. This creates a very practical window into which testing modality may best represent systemic T exposure when it is administered via the skin.
If you are new to this controversy, you might think this is a ridiculous question. Thanks to the extremely exaggerated values in saliva and dried blood spot (DBS) when testosterone therapy is given as a transdermal cream or gel, this topic is highly relevant and historically has been quite confusing. The data in this blog will help clear up some of the confusion.
Why is there a controversy? Saliva and DBS are thought to correlate reasonably well with serum or urine testing when men are not taking exogenous hormones. When a transdermal testosterone gel or cream is applied to the skin, the increased values observed in saliva and DBS greatly exceed the response in serum or urine. Some proponents of saliva and DBS testing claim that they better represent tissue exposure in this scenario. I once believed this to be true; but after years of reviewing test results along with clinical responses, as well as a deep dive into the literature, I realize this is not true.
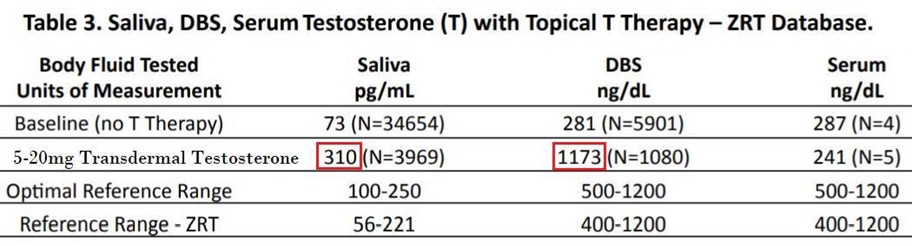
Saliva (along with DBS, serum, and urine) results for patients using TD T were recently published (not a peer-reviewed publication) for consideration. I want to draw your attention to the increase in saliva and DBS concentrations in men using TD T 5-20mg. Saliva T results average 310pg/mL (much higher than the reference range for men, which is 56-221pg/mL) and DBS T results average 1173 (high normal, reference range is 400-1200ng/dL). Both would lead you to believe that TD T 5-20mg may be too much for a man. Serum results in this publication are only based on five values, but they are consistent with the literature (25-50mg is usually the minimal dose to increase levels significantly in men).1,2,3,4 This dichotomy (very high results in saliva/DBS and much lower results in serum/urine) is not disputed. What is disputed is the interpretation of these results.
Saliva testing tells us that the average man with testosterone deficiency on TD T 5-20mg has a value higher than the male reference range. These samples were believed to have been collected ~ 24 hours after a TD T dose; so, this represents the lowest point before the next dose. If measured about 12 hours after supplementation the values are 2-3 times higher and even higher yet between 4-8 hours after a dose. These concentrations convey the message that the saliva gland is full of lots of testosterone. Serum and urine levels don’t increase significantly in men taking these 5-20mg doses. This is a huge discrepancy!
I have talked much about this in the past and provided a lot of evidence from the literature suggesting that clinical changes in men align more with serum/urine responses. Changes in LH, muscle mass, sexual function, estrogen and DHT production, and increases in bone mineral density and erythrocytosis, etc. all seem to parallel changes in serum for men on TD T therapy.1-4,5,6 Doses of > 25mg are required for clinical success in men. None of these clinical measurements show the type of response expected if these doses are mega-doses as saliva and DBS testing implies.
THINK ABOUT IT: Increases in erythrocytosis from a typical injection are far more than those observed when using TD T 50 or 100mg (AndroGel). This is a good example of clinical changes aligning with the modest increases seen in serum or urine when using 50-100mg TD T, doses that increase saliva and DBS 10-fold above baseline. If this dramatic increase represented tissue exposure, erythrocytosis would unsafely increase with these doses.6
I want to add one very practical clinical question to this debate. What happens when women take TD T 5-20mg?
If saliva testing “speaks” for tissue exposure, 5-20mg may be too much T for a man! If saliva best represents the clinical reality, we’d expect women taking TD T 5-20mg to regularly report symptoms of excess androgen exposure. Do they? If changes in serum and urine better represent clinical realities, we’d expect women on these doses to potentially eliminate female androgen deficiency symptoms, but we would not necessarily expect them to report symptoms associated with too much testosterone. So, what does the scientific literature have to say? So glad you asked!
At least ten different published studies have been identified in which women were treated with TD T 5-30mg (gels, creams, or sprays).7,8,9,10,11,12,13,14,15,16,17 In many of these studies, validated surveys of signs of hirsutism (Ferriman-Gallwey scale) and acne (Palatsi scale) were used, and evaluations of any voice deepening, or other signs of androgen excess were performed. None of these studies reported ANY signs of excess testosterone. You might want to read that again – EVERY study published using TD T 5-30mg daily to treat females resulted in NO REPORTED symptoms of excess androgens.
What the studies did report was a moderate increase in serum testosterone, as expected (see below to see DUTCH data showing similarly modest, yet significant increases with these same doses). Most studies observed testosterone levels approximately doubling from low or low normal results increasing to within normal ranges. For example, El-Hage reported levels below a given range of 0-2.1nmol/L (0-60ng/dL, reference range < 2.6nmol/L or < 75ng/dL) at baseline. Serum levels increased to 3.8nmol/L (110ng/dL) after three months of testosterone cream therapy. Accompanying these serum increases were improvements in sexuality scores10 self-reported satisfactory sexual events,9 psychological general well-being index, Sabbatsberg sexual self-rating scale, and other objective measures of improved outcomes related to eliminating androgen deficiencies in female patients.11
DON’T MISS THE POINT: We are not arguing in this piece for any particular TD T dose to be used in treating androgen deficient women. We are simply making the point that TD T 5-20mg has been shown to NOT cause high T symptoms in women, which is illuminating in the testing debate.
So, what do we learn from these studies?
Studies of male patients taking TD T have implied, without exception, that salivary (and DBS) values are exaggerated far above the clinical response. Studies of female patients on similar products confirm that TD T 5-20mg certainly cannot be excessive for male patients. Saliva and DBS results are believed to be legitimately increased to supraphysiological levels, but these observations are, to date, without clinical significance and should never be used to monitor treatment. It was our attempt to be comprehensive in surveying the scientific literature.
We welcome any studies that may contradict our conclusions from the studies listed here. For example, a study resulting in low or normal serum levels in women and high androgen symptoms using TD T 5-20mg would bend the debate in the direction of salivary testing proponents. Given the current state of peer-reviewed research on this topic, there doesn’t seem to be a justified reason to use saliva or DBS measurements to monitor these types of therapies. If clinical success is the goal, these testing modalities are counterproductive to ensuring positive clinical outcomes.
What about urine testosterone (DUTCH) measurements?
For monitoring transdermal estradiol (patches, gels, or creams) in women, we consider the DUTCH test the best option. Up-and-down patterns that may be seen in serum with gels and creams can be smoothed out with a collection that represents 12-16 hours of the day as compared to a serum test that represents just a few seconds or minutes. For patches, gels, and creams, DUTCH levels scale up intuitively as the dosing increases. Additionally, monitoring metabolites adds value to the testing. Importantly, DUTCH estradiol measurements are highly accurate, and published data show strong correlation with serum measurements.18
REMEMBER: Some studies evaluated total testosterone (T),10 while others monitored free or bioavailable T,9 similar to the DUTCH test. Regardless of whether the measure was total T, free T, or bioavailable T, the results were similar. When TD T is applied, serum free T increases in parallel with serum total T. The fact that free T is measured in saliva does not explain the high amount of T that concentrates in the salivary gland with TD T use.
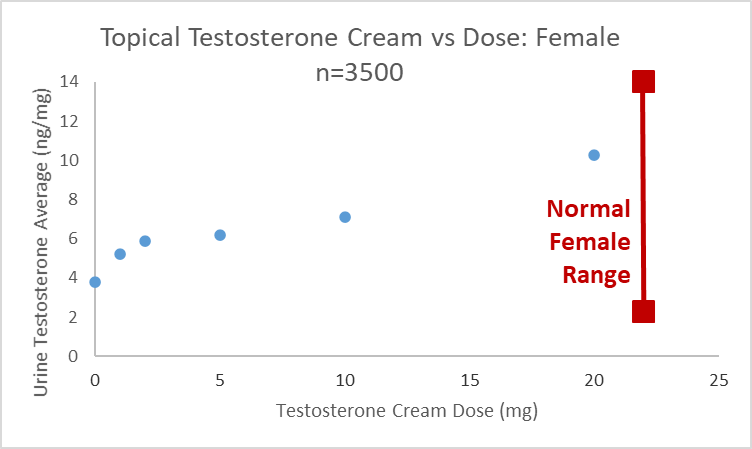
Using urine to monitor testosterone is somewhat less reliable when compared to estrogen. We recently published validation data for DUTCH measurements, including 8 different androgens. These values correlate well to 24-hour collections and are analytically reliable.19 Data informally analyzed shows that DUTCH results do show an increase in patients taking TD T creams. As the graph below shows, the average (median) values were within the normal range (2.3-14ng/mg) as has been reported in serum. We found that just over 1/3 of female patients taking 20mg showed elevated DUTCH testosterone results while those taking 10mg had elevated results in about 1/5 of patients.
As with estrogens, the up-and-down pattern of testosterone throughout the day can be nicely averaged out using the DUTCH test.18,19 Despite its strengths, urine testing does have a notable weakness for testosterone. The correlation with serum is not believed to be as consistently reliable for testosterone.
All sex hormones measured in urine are found in their conjugated forms. These phase 2 metabolites (glucuronide and sulfate forms) are thought to represent bioavailable hormone, but the testing assumes consistently proper phase 2 metabolism. This is not always true of testosterone. There are known variants in the enzyme responsible for conjugating testosterone (UGT 2B17).20,21 These variants can result in artificially low urine testosterone when serum levels are not low. This issue is especially prevalent in the southeast Asian population and exists in a minority of other ethnic groups. The bottom line: low urinary testosterone can be caused by testosterone deficiency. However, low urinary testosterone results may occur in testosterone sufficient men and women who aren’t able to conjugate testosterone (make testosterone water soluble), because of the UGT SNP.
Serum is still considered the gold standard for monitoring testosterone therapy, including TD T. We consider DUTCH a first-line test for TD estradiol and a complimentary test for monitoring TD testosterone. DUTCH allows the examination of testosterone metabolism and answering questions like the following:
- How is the body metabolizing testosterone?
- Are more active, potent 5a metabolites like 5a-DHT preferred or not?
- How much estrogen is being made from testosterone (testosterone is metabolized directly to estradiol by the aromatase enzyme)
- Of the available estrogens, how are they metabolized – Phase 1 (2-OH, 4-OH, 16-OH estrogens)
- Of the available 2-OH and 4-OH estrogens, how well are they methylated via phase 2 metabolism?
- DUTCH also allows for the examination of the HPA axis and other important measurements that impact testosterone and its clinical efficacy but may not be directly related to testosterone.
For practitioners wanting to comprehensively assess patients on TD T therapy, we recommend serum and DUTCH testing.
References
[1] Wang C, et al. Transdermal Testosterone Gel Improves Sexual Function, Mood, Muscle Strength, and Body Composition Parameters in Hypogonadal Men. J Clin Endocrinol Metab. 2000; 85(8): 2839-2853. https://doi.org/10.1210/jc.85.8.2839.
[2] Brockenbrough AT, et al. Transdermal Androgen Therapy to Augment EPO in the Treatment of Anemia of Chronic Renal Disease. Amer J Kidney Dis. 2006; 47(2): 251-262. https://doi.org/10.1053/j.ajkd.2005.10.022.
[3] Wang C, et al. Long-Term Testosterone Gel (AndroGel) Treatment Maintains Beneficial Effects on Sexual Function and Mood, Lean and Fat Mass, and Bone Mineral Density in Hypogonadal Men. J Clin Endocrinol Metab. 2004; 89(5): 2085-2098. doi: 10.1210/jc.2003-032006
[4] Sattler F, et al. Testosterone Threshold Levels and Lean Tissue Mass Targets Needed to Enhance Skeletal Muscle Strength and Function: The HORMA Trial. J Gerontol A Biol Sci Med Sci. 2011; 66(1): 122-129. https://doi.org/10.1093/gerona/glq183.
[5] Swerdloff RS, et al. Long-Term Pharmacokinetics of Transdermal Testosterone Gel in Hypogonadal Men. J Clin Endocrinol Metab. 2000; 85(12): 4500-4510. https://doi.org/10.1210/jc.85.12.4500.
[6] Ohlander SJ, et al. Erythrocytosis Following Testosterone Therapy. Sex Med Rev. 2018; 6(1): 77-85. https://doi.org/10.1016/j.sxmr.2017.04.001.
[7] Barton DL, et al. Randomized Controlled Trial to Evaluate Transdermal Testosterone in Female Cancer Survivors With Decreased Libido; North Central Cancer Treatment Group Protocol N02C3. J Natl Cancer Inst. 2007; 99(9): 672-679. doi: 10.1093/jnci/djk149.
[8] Chaikittisilpa S, et al. Efficacy of oral estrogen plus testosterone gel to improve sexual function in postmenopausal women. Climacteric. 2019; 22(5): 460-465. doi: 10.1080/13697137.2019.1577378.
[9] Davis S, et al. Safety and Efficacy of a Testosterone Metered-Dose Transdermal Spray for Treating Decreased Sexual Satisfaction in Premenopausal Women. Ann Intern Med. 2008; 148(8): 569-577. doi: 10.7326/0003-4819-148-8-200804150-00001.
[10] El-Hage G, et al. A double-blind, randomized, placebo-controlled trial of the effect of testosterone cream on the sexual motivation of menopausal hysterectomized women with hypoactive sexual desire disorder. Climacteric. 2007; 10(4): 335-343. doi: 10.1080/13697130701364644.
[11] Goldstat R, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003; 10(5): 390-398. https://doi.org/10.1097/01.gme.0000060256.03945.20
[12] Kim C, et al. Ovarian Features after 2 Weeks, 3 Weeks and 4 Weeks Transdermal Testosterone Gel Treatment and Their Associated Effect on IVF Outcomes in Poor Responders. Dev Reprod. 2014; 18(3): 145-152. doi: 10.12717/DR.2014.18.3.145.
[13] Nathorst-Böös J, et al. Percutaneous administration of testosterone gel in postmenopausal women – a pharmacological study. Gynecol Endocrinol. 2005; 20(5): 243-248. doi: 10.1080/09513590500097283.
[14] Smith GI, et al. Systemic Delivery of Estradiol, but not Testosterone or Progesterone, Alters Very Low Density Lipoprotein-Triglyceride Kinetics in Postmenopausal Women. J Clin Endocrinol Metab. 2014; 99(7): E1306-1310. doi: 10.1210/jc.2013-4470.
[15] Waldman T, et al. Safety and efficacy of transdermal testosterone for treatment of hypoactive sexual desire disorder. Clin Invest. 2012; 2(4): 423-432.
[16] Pelusi C, et al. Effects of Three Different Testosterone Formulations in Female‐to‐Male Transsexual Persons. J Sex Med. 2014; 11(12): 3002-3011. doi: 10.1111/jsm.12698.
[17] Urman B, et al. Elevated Serum Testosterone, Hirsutism, and Virilism Associated with Combined Androgen-Estrogen Hormone Replacement Therapy. Obstet Gynecol. 1991; 77(4): 595-598.
[18] Newman M, et al. Evaluating urinary estrogen and progesterone metabolites using dried filter paper samples and gas chromatography with tandem mass spectrometry (GC/MS-MS). BMC Chem. 2019; 13(1): 20. doi: 10.1186/s13065-019-0539-1.
[19] Newman M, Curran DA. Reliability of a dried urine test for comprehensive assessment of urine hormones and metabolites. BMC Chem. 2021; 15(1): 8. doi: 10.1186/s13065-021-00744-3.
[20] Jakobsson J, et al. Large Differences in Testosterone Excretion in Korean and Swedish Men Are Strongly Associated with a UDP-Glucuronosyl Transferase 2B17 Polymorphism. J Clin Endocrinol Metab. 2006; 91(2): 687-693. doi: 10.1210/jc.2005-1643.
[21] Sten T, et al. UDP-Glucuronosyltransferases (UGTs) 2B7 and UGT2B17 Display Converse Specificity in Testosterone and Epitestosterone Glucuronidation, whereas UGT2A1 Conjugates Both Androgens Similarly. Drug Metab Dispos. 2009; 37(2): 417-423. doi: 10.1124/dmd.108.024844.